Predictive significance of the proportion of ER-positive or PgR-positive tumor cells in response to neoadjuvant chemotherapy for operable HER2-negative breast cancer
- PMID: 22969846
- PMCID: PMC3438790
- DOI: 10.3892/etm.2011.359
Predictive significance of the proportion of ER-positive or PgR-positive tumor cells in response to neoadjuvant chemotherapy for operable HER2-negative breast cancer
Abstract
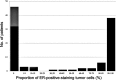
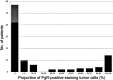
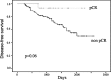
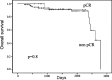
Estrogen receptor (ER) and progesterone receptor (PgR) status are predictive factors for the clinical and pathological response to neoadjuvant chemotherapy for operable breast cancer. However, it remains unclear as to how the proportion of ER-positive or PgR-positive tumor cells affects the response to neoadjuvant chemotherapy. We examined the correlation of the proportion of ER-positive or PgR-positive tumor cells with the clinical and pathological response to neoadjuvant chemotherapy for operable human epidermal growth factor receptor 2 (HER2)-negative breast cancer. From April 2002 to October 2010, 103 patients received neoadjuvant chemotherapy containing epirubicin and taxane in our clinic. A clinical response was observed in 86 (83%) patients, and a pathological complete response (pCR) was observed in 16 (16%) patients. Fourteen (30%) of 46 patients with ER-negative tumors achieved pCR and 15 (26%) of 57 patients with PgR-negative tumors achieved pCR. Patients with more than 30% ER-positive tumor cells or more than 1% PgR-positive tumor cells did not achieve pCR. No significant correlation was observed between pCR and the menopausal status, tumor size, grade and Ki-67 expression. In univariate analysis, pCR was associated with the ER status (p=0.001), PgR status (p=0.0001) and chemotherapy regimens (p=0.03). Multivariate analysis revealed that ER and PgR status were significant factors for pCR, and patients with ER-negative tumors were 18.6 times more likely to achieve pCR than those with greater than or equal to 30% ER-positive tumor cells (p=0.006; 95% confidence interval 2.3-149.9). We demonstrated a predictive significance of the proportion of ER-positive or PgR-positive tumor cells in the response to neoadjuvant chemotherapy for operable HER2-negative breast cancer. ER-negativity (<1%) was a significant predictive factor for achieving pCR in multivariate analysis. Conversely, patients with more than 30% ER-positive tumor cells or more than 1% PgR-positive tumor cells may not achieve pCR.
Figures


Similar articles
-
Outcome after neoadjuvant chemotherapy in estrogen receptor-positive and progesterone receptor-negative breast cancer patients: a pooled analysis of individual patient data from ten prospectively randomized controlled neoadjuvant trials.Breast Cancer Res Treat. 2018 Jan;167(1):59-71. doi: 10.1007/s10549-017-4480-5. Epub 2017 Sep 5. Breast Cancer Res Treat. 2018. PMID: 28875243
-
Evaluation of ER, PgR, HER-2, Ki-67, cyclin D1, and nm23-H1 as predictors of pathological complete response to neoadjuvant chemotherapy for locally advanced breast cancer.Med Oncol. 2011 Dec;28 Suppl 1:S31-8. doi: 10.1007/s12032-010-9676-z. Epub 2010 Sep 16. Med Oncol. 2011. PMID: 20844986
-
Factors Affecting Pathological Complete Response After Neoadjuvant Chemotherapy in Operable Primary Breast Cancer.J Coll Physicians Surg Pak. 2020 Apr;30(4):389-393. doi: 10.29271/jcpsp.2020.04.389. J Coll Physicians Surg Pak. 2020. PMID: 32513358
-
Analysis of the pathologic response to primary chemotherapy in patients with locally advanced breast cancer grouped according to estrogen receptor, progesterone receptor, and HER2 status.Clin Breast Cancer. 2007 Apr;7(7):559-64. doi: 10.3816/CBC.2007.n.012. Clin Breast Cancer. 2007. PMID: 17509165 Clinical Trial.
-
Lessons on responsiveness to adjuvant systemic therapies learned from the neoadjuvant setting.Breast. 2009 Oct;18 Suppl 3:S137-40. doi: 10.1016/S0960-9776(09)70289-9. Breast. 2009. PMID: 19914533 Review.
Cited by
-
The relationship between NFKB, HER2, ER expression and anthracycline -based neoadjuvan chemotherapy response in local advanced stadium breast cancer: A cohort study in Eastern Indonesia.Ann Med Surg (Lond). 2021 Feb 10;63:102164. doi: 10.1016/j.amsu.2021.02.010. eCollection 2021 Mar. Ann Med Surg (Lond). 2021. PMID: 33664949 Free PMC article.
-
Evaluation of hormone receptor, human epidermal growth factor receptor-2 and Ki-67 with core needle biopsy and neoadjuvant chemotherapy effects in breast cancer patients.Thorac Cancer. 2015 Jan;6(1):64-9. doi: 10.1111/1759-7714.12133. Epub 2015 Jan 7. Thorac Cancer. 2015. PMID: 26273337 Free PMC article.
-
Development and Assessment of a Novel Core Biopsy-Based Prediction Model for Pathological Complete Response to Neoadjuvant Chemotherapy in Women with Breast Cancer.Int J Environ Res Public Health. 2023 Jan 16;20(2):1617. doi: 10.3390/ijerph20021617. Int J Environ Res Public Health. 2023. PMID: 36674372 Free PMC article.
References
-
- Van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. - PubMed
-
- Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from the National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. - PubMed
-
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. - PubMed
-
- Makris A, Powles TJ, Ashley SE, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol. 1998;9:1179–1184. - PubMed
-
- Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS) Ann Oncol. 1999;10:47–52. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
