Polyethylene glycol 4000 treatment for children with constipation: A randomized comparative multicenter study
- PMID: 22969980
- PMCID: PMC3438798
- DOI: 10.3892/etm.2012.491
Polyethylene glycol 4000 treatment for children with constipation: A randomized comparative multicenter study
Abstract
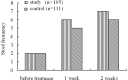
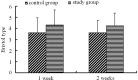
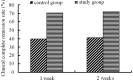
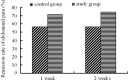
The aim of this study was to evaluate the efficacy and safety of polyethylene glycol 4000 (PEG 4000) for the treatment of constipation in children over 8 years of age. A total of 216 children from 7 hospitals were enrolled. A total of 105 patients received oral PEG 4000 (20 g/day) and 111 patients received oral lactulose (15 ml/day) for 2 weeks. The stool frequency, stool consistency and abdominal pain of the patients were monitored. In the PEG group, following one week and two weeks of treatment, the median weekly stool frequency improved from 2 times prior to treatment to 6 and 7 times, respectively, following treatment. The clinical remission rates of the PEG and lactulose groups following one week of treatment were 70.48 and 39.64%, respectively, and following two weeks of treatment were 72.38 and 41.44%, respectively. Abdominal pain disappeared in 74.6% of patients following two weeks of PEG 4000 treatment. No significant clinical adverse effects or abnormalities in the laboratory tests were observed in the two treatment groups. In conclusion, PEG 4000 is a safe and more effective drug compared to lactulose for the treatment of constipation in children.
Figures
References
-
- Wang MG, Wang BX. Initial therapy of constipation in children. J Appl Clin Pediatr. 2006;21:446–448.
-
- Corazziari E, Badiali D, Habib FI, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in treatment of chronic nonorganic constipation. Dig Dis Sci. 1996;41:1636–1642. - PubMed
-
- DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95:446–450. - PubMed
LinkOut - more resources
Full Text Sources
