Phosphodiesterase 4 regulates the migration of B16-F10 melanoma cells
- PMID: 22970026
- PMCID: PMC3439151
- DOI: 10.3892/etm.2012.587
Phosphodiesterase 4 regulates the migration of B16-F10 melanoma cells
Abstract
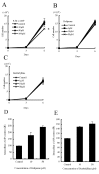
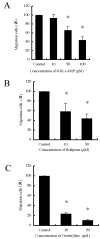
Phosphodiesterases (PDEs) are important regulators of signal transduction processes. Eleven PDE gene families (PDE1-11) have been identified and several PDE isoforms are selectively expressed in various cell types. PDE4 family members specifically hydrolyze cyclic AMP (cAMP). Four genes (PDE4A-D) are known to encode PDE4 enzymes, with additional diversity generated by the use of alternative mRNA splicing and the use of different promoters. While PDE4 selective inhibitors show therapeutic potential for treating major diseases such as asthma and chronic obstructive pulmonary disease, little is known concerning the role of PDE4 in malignant melanoma. In this study, we examined the role of PDE4 in mouse B16-F10 melanoma cells. In these cells, PDE4 activity was found to be ∼60% of total PDE activity. RT-PCR detected only PDE4B and PDE4D mRNA. Cell growth was inhibited by the cAMP analog, 8-bromo-cAMP, but not by the specific PDE4 inhibitors, rolipram and denbufylline, which increased intracellular cAMP concentrations. Finally, migration of the B16-F10 cells was inhibited by the PDE4 inhibitors and 8-bromo-cAMP, while migration was increased by a protein kinase A (PKA) inhibitor, PKI(14-22), and was not affected by 8-pCPT-2'-O-Me-cAMP, which is an analog of exchange protein activated by cAMP (Epac). The inhibitory effect of rolipram on migration was reversed by PKI(14-22). Based on these results, PDE4 appears to play an important role in the migration of B16-F10 cells, and therefore may be a novel target for the treatment of malignant melanoma.
Figures



References
-
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;36:687–701. - PubMed
-
- Eggermont AM, Robert C. New drugs in melanoma: it’s a whole new world. Eur J Cancer. 2011;47:2150–2157. - PubMed
-
- Beavo JA, Houslay MD, Francis SH. Cyclic nucleotide phosphodiesterase superfamily. In: Beavo JA, Francis SH, Hously MD, editors. Cyclic Nucleotide Phosphodiesterases In Health and Disease. CRC Press; New York: 2007. pp. 3–17.
-
- Hously MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. - PubMed
-
- Hously MD. Underpinning compartmentalized cAMP signaling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
