Mapping the mechanome of live stem cells using a novel method to measure local strain fields in situ at the fluid-cell interface
- PMID: 22970134
- PMCID: PMC3438189
- DOI: 10.1371/journal.pone.0043601
Mapping the mechanome of live stem cells using a novel method to measure local strain fields in situ at the fluid-cell interface
Abstract
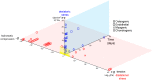
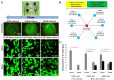
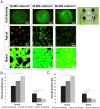
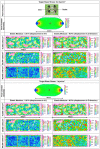
During mesenchymal condensation, the initial step of skeletogenesis, transduction of minute mechanical forces to the nucleus is associated with up or down-regulation of genes, ultimately resulting in formation of the skeletal template and appropriate cell lineage commitment. The summation of these biophysical cues affects the cell's shape and fate. Here, we predict and measure surface strain, in live stem cells, in response to controlled delivery of stresses, providing a platform to direct short-term structure--function relationships and long-term fate decisions. We measure local strains on stem cell surfaces using fluorescent microbeads coated with Concanavalin A. During delivery of controlled mechanical stresses, 4-Dimensional (x,y,z,t) displacements of the bound beads are measured as surface strains using confocal microscopy and image reconstruction. Similarly, micro-particle image velocimetry (μ-piv) is used to track flow fields with fluorescent microspheres. The measured flow velocity gradient is used to calculate stress imparted by fluid drag at the surface of the cell. We compare strain measured on cell surfaces with those predicted computationally using parametric estimates of the cell's elastic and shear modulus. Finally, cross-correlating stress--strain data to measures of gene transcription marking lineage commitment enables us to create stress--strain--fate maps, for live stem cells in situ. The studies show significant correlations between live stem cell stress--strain relationships and lineage commitment. The method presented here provides a novel means to probe the live stem cell's mechanome, enabling mechanistic studies of the role of mechanics in lineage commitment as it unfolds.
Conflict of interest statement
Figures



References
-
- Knothe Tate ML, Dolejs S, Miller RM, Knothe UR (2010) Role of mechanical loading in healing of massive bone autografts. J Orthop Res 28: 1657–1664. - PubMed
-
- Zimmerman J, Knothe Tate ML (2011) Structure - function relationships in the stem cell's mechanical world A: Seeding protocols as a means to control shape and fate in live cells. Mol Cell Biomech 8: 275–296. - PubMed
-
- Anderson EJ, Knothe Tate ML (2007) Design of tissue engineering scaffolds as delivery devices for mechanical and mechanically modulated signals. Tissue Eng 13: 2525–2538. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

