Novel levamisole derivative induces extrinsic pathway of apoptosis in cancer cells and inhibits tumor progression in mice
- PMID: 22970136
- PMCID: PMC3438185
- DOI: 10.1371/journal.pone.0043632
Novel levamisole derivative induces extrinsic pathway of apoptosis in cancer cells and inhibits tumor progression in mice
Abstract
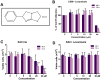
Background: Levamisole, an imidazo(2,1-b)thiazole derivative, has been reported to be a potential antitumor agent. In the present study, we have investigated the mechanism of action of one of the recently identified analogues, 4a (2-benzyl-6-(4'-fluorophenyl)-5-thiocyanato-imidazo[2,1-b][1], [3], [4]thiadiazole).
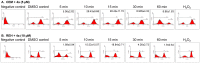
Materials and methods: ROS production and expression of various apoptotic proteins were measured following 4a treatment in leukemia cell lines. Tumor animal models were used to evaluate the effect of 4a in comparison with Levamisole on progression of breast adenocarcinoma and survival. Immunohistochemistry and western blotting studies were performed to understand the mechanism of 4a action both ex vivo and in vivo.
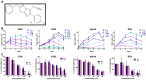
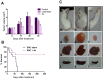
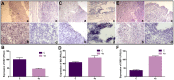
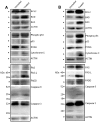
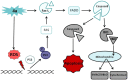
Results: We have determined the IC(50) value of 4a in many leukemic and breast cancer cell lines and found CEM cells most sensitive (IC(50) 5 µM). Results showed that 4a treatment leads to the accumulation of ROS. Western blot analysis showed upregulation of pro-apoptotic proteins t-BID and BAX, upon treatment with 4a. Besides, dose-dependent activation of p53 along with FAS, FAS-L, and cleavage of CASPASE-8 suggest that it induces death receptor mediated apoptotic pathway in CEM cells. More importantly, we observed a reduction in tumor growth and significant increase in survival upon oral administration of 4a (20 mg/kg, six doses) in mice. In comparison, 4a was found to be more potent than its parental analogue Levamisole based on both ex vivo and in vivo studies. Further, immunohistochemistry and western blotting studies indicate that 4a treatment led to abrogation of tumor cell proliferation and activation of apoptosis by the extrinsic pathway even in animal models.
Conclusion: Thus, our results suggest that 4a could be used as a potent chemotherapeutic agent.
Conflict of interest statement
Figures


References
-
- Nambiar M, Kari V, Raghavan SC (2008) Chromosomal translocations in cancer. Biochim Biophys Acta 1786: 139–152. - PubMed
-
- Aplan PD (2005) “You break it, you fix it.”. Blood 105: 1843–1844. - PubMed
-
- Rowley JD (2001) Chromosome translocations: dangerous liaisons revisited. Nat Rev Cancer 1: 245–250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

