Optimisation of prime-boost immunization in mice using novel protein-based and recombinant vaccinia (Tiantan)-based HBV vaccine
- PMID: 22970140
- PMCID: PMC3435326
- DOI: 10.1371/journal.pone.0043730
Optimisation of prime-boost immunization in mice using novel protein-based and recombinant vaccinia (Tiantan)-based HBV vaccine
Abstract
Background: A therapeutic vaccine for chronic hepatitis B virus (HBV) infection that enhances virus-specific cellular immune responses is urgently needed. The "prime-boost" regimen is a widely used vaccine strategy against many persistence infections. However, few reports have addressed this strategy applying for HBV therapeutic vaccine development.
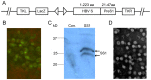
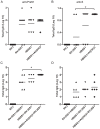
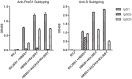
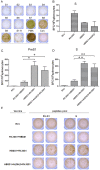
Methodology/principal findings: To develop an effective HBV therapeutic vaccine, we constructed a recombinant vaccinia virus (Tiantan) containing the S+PreS1 fusion antigen (RVJSS1) combined with the HBV particle-like subunit vaccine HBVSS1 to explore the most effective prime-boost regimen against HBV. The immune responses to different prime-boost regimens were assessed in C57BL/C mice by ELISA, ELISpot assay and Intracellular cytokine staining analysis. Among the combinations tested, an HBV protein particle vaccine priming and recombinant vaccinia virus boosting strategy accelerated specific seroconversion and produced high antibody (anti-PreS1, anti-S antibody) titres as well as the strongest multi-antigen (PreS1, and S)-specific cellular immune response. HBSS1 protein prime/RVJSS1 boost immunization was also generated more significant level of both CD4+ and CD8+ T cell responses for Th1 cytokines (TNF-α and IFN-γ).
Conclusions: The HBSS1 protein-vaccine prime plus RVJSS1 vector boost elicits specific antibody as well as CD4 and CD8 cells secreting Th1-like cytokines, and these immune responses may be important parameters for the future HBV therapeutic vaccines.
Conflict of interest statement
Figures


References
-
- Ganem D, Prince AM (2004) Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 350: 1118–1129. - PubMed
-
- Michel ML, Tiollais P (2010) Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol 58: 288–295. - PubMed
-
- Sheppard CW, Samar EP, Finely L, Fiore AE, Bell BP (2006) Hepatitis B virus infection: epidemiology and vaccination. Epidemiology Rev 28: 112–125. - PubMed
-
- Chen HL, Chang MH, Ni YH, Hsu HY, Lee PI, et al. (1996) Seroepidemiology of hepatitis B virus infection in children: ten years of mass vaccination in Taiwan. JAMA 276: 906–908. - PubMed
-
- Zhou YH, Wu C, Zhuang H (2009) Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl) 122: 98–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

