Within-subject interlaboratory variability of QuantiFERON-TB gold in-tube tests
- PMID: 22970142
- PMCID: PMC3435391
- DOI: 10.1371/journal.pone.0043790
Within-subject interlaboratory variability of QuantiFERON-TB gold in-tube tests
Abstract
Background: The QuantiFERON®-TB Gold In-Tube test (QFT-GIT) is a viable alternative to the tuberculin skin test (TST) for detecting Mycobacterium tuberculosis infection. However, within-subject variability may limit test utility. To assess variability, we compared results from the same subjects when QFT-GIT enzyme-linked immunosorbent assays (ELISAs) were performed in different laboratories.
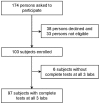
Methods: Subjects were recruited at two sites and blood was tested in three labs. Two labs used the same type of automated ELISA workstation, 8-point calibration curves, and electronic data transfer. The third lab used a different automated ELISA workstation, 4-point calibration curves, and manual data entry. Variability was assessed by interpretation agreement and comparison of interferon-γ (IFN-γ) measurements. Data for subjects with discordant interpretations or discrepancies in TB Response >0.05 IU/mL were verified or corrected, and variability was reassessed using a reconciled dataset.
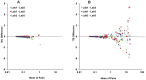
Results: Ninety-seven subjects had results from three labs. Eleven (11.3%) had discordant interpretations and 72 (74.2%) had discrepancies >0.05 IU/mL using unreconciled results. After correction of manual data entry errors for 9 subjects, and exclusion of 6 subjects due to methodological errors, 7 (7.7%) subjects were discordant. Of these, 6 (85.7%) had all TB Responses within 0.25 IU/mL of the manufacturer's recommended cutoff. Non-uniform error of measurement was observed, with greater variation in higher IFN-γ measurements. Within-subject standard deviation for TB Response was as high as 0.16 IU/mL, and limits of agreement ranged from -0.46 to 0.43 IU/mL for subjects with mean TB Response within 0.25 IU/mL of the cutoff.
Conclusion: Greater interlaboratory variability was associated with manual data entry and higher IFN-γ measurements. Manual data entry should be avoided. Because variability in measuring TB Response may affect interpretation, especially near the cutoff, consideration should be given to developing a range of values near the cutoff to be interpreted as "borderline," rather than negative or positive.
Conflict of interest statement
Figures
References
-
- Denkinger CM, Dheda K, Pai M (2011) Guidelines on interferon-gamma release assays for tuberculosis infection: concordance, discordance or confusion? Clin Microbiol Infect 17: 806–814. - PubMed
-
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, et al. (2010) Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 59: 1–25. - PubMed
-
- Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, et al. (2010) Interferon-{gamma} release assays for the diagnosis of latent M. tuberculosis infection: A systematic review and meta-analysis. Eur Respir J 37: 88–99. - PubMed
-
- Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, et al. (2006) Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis . Am J Respir Crit Care Med 174: 831–839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous