Exploring the mechanism of zanamivir resistance in a neuraminidase mutant: a molecular dynamics study
- PMID: 22970161
- PMCID: PMC3435372
- DOI: 10.1371/journal.pone.0044057
Exploring the mechanism of zanamivir resistance in a neuraminidase mutant: a molecular dynamics study
Abstract
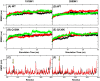
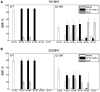
It is critical to understand the molecular basis of the drug resistance of influenza viruses to efficiently treat this infectious disease. Recently, H1N1 strains of influenza A carrying a mutation of Q136K in neuraminidase were found. The new strain showed a strong Zanamivir neutralization effect. In this study, normal molecular dynamics simulations and metadynamics simulations were employed to explore the mechanism of Zanamivir resistance. The wild-type neuraminidase contained a 3(10) helix before the 150 loop, and there was interaction between the 150 and 430 loops. However, the helix and the interaction between the two loops were disturbed in the mutant protein due to interaction between K136 and nearby residues. Hydrogen-bond network analysis showed weakened interaction between the Zanamivir drug and E276/D151 on account of the electrostatic interaction between K136 and D151. Metadynamics simulations showed that the free energy landscape was different in the mutant than in the wild-type neuraminidase. Conformation with the global minimum of free energy for the mutant protein was different from the wild-type conformation. While the drug fit completely into the active site of the wild-type neuraminidase, it did not match the active site of the mutant variant. This study indicates that the altered hydrogen-bond network and the deformation of the 150 loop are the key factors in development of Zanamivir resistance. Furthermore, the Q136K mutation has a variable effect on conformation of different N1 variants, with conformation of the 1918 N1 variant being more profoundly affected than that of the other N1 variants studied in this paper. This observation warrants further experimental investigation.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

