Expression of MUC17 is regulated by HIF1α-mediated hypoxic responses and requires a methylation-free hypoxia responsible element in pancreatic cancer
- PMID: 22970168
- PMCID: PMC3438193
- DOI: 10.1371/journal.pone.0044108
Expression of MUC17 is regulated by HIF1α-mediated hypoxic responses and requires a methylation-free hypoxia responsible element in pancreatic cancer
Abstract
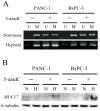
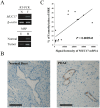
MUC17 is a type 1 membrane-bound glycoprotein that is mainly expressed in the digestive tract. Recent studies have demonstrated that the aberrant overexpression of MUC17 is correlated with the malignant potential of pancreatic ductal adenocarcinomas (PDACs); however, the exact regulatory mechanism of MUC17 expression has yet to be identified. Here, we provide the first report of the MUC17 regulatory mechanism under hypoxia, an essential feature of the tumor microenvironment and a driving force of cancer progression. Our data revealed that MUC17 was significantly induced by hypoxic stimulation through a hypoxia-inducible factor 1α (HIF1α)-dependent pathway in some pancreatic cancer cells (e.g., AsPC1), whereas other pancreatic cancer cells (e.g., BxPC3) exhibited little response to hypoxia. Interestingly, these low-responsive cells have highly methylated CpG motifs within the hypoxia responsive element (HRE, 5'-RCGTG-3'), a binding site for HIF1α. Thus, we investigated the demethylation effects of CpG at HRE on the hypoxic induction of MUC17. Treatment of low-responsive cells with 5-aza-2'-deoxycytidine followed by additional hypoxic incubation resulted in the restoration of hypoxic MUC17 induction. Furthermore, DNA methylation of HRE in pancreatic tissues from patients with PDACs showed higher hypomethylation status as compared to those from non-cancerous tissues, and hypomethylation was also correlated with MUC17 mRNA expression. Taken together, these findings suggested that the HIF1α-mediated hypoxic signal pathway contributes to MUC17 expression, and DNA methylation of HRE could be a determinant of the hypoxic inducibility of MUC17 in pancreatic cancer cells.
Conflict of interest statement
Figures




References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. (2009) Cancer statistics, 2009. CA: a cancer journal for clinicians 59: 225–249. - PubMed
-
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nature reviews Cancer 3: 721–732. - PubMed
-
- Bhaskar KR, Garik P, Turner BS, Bradley JD, Bansil R, et al. (1992) Viscous fingering of HCl through gastric mucin. Nature 360: 458–461. - PubMed
-
- Ho SB, Dvorak LA, Moor RE, Jacobson AC, Frey MR, et al. (2006) Cysteine-rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology 131: 1501–1517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

