A novel in vivo siRNA delivery system specifically targeting liver cells for protection of ConA-induced fulminant hepatitis
- PMID: 22970170
- PMCID: PMC3435394
- DOI: 10.1371/journal.pone.0044138
A novel in vivo siRNA delivery system specifically targeting liver cells for protection of ConA-induced fulminant hepatitis
Abstract
Background: Fulminant hepatitis progresses to acute liver failure (ALF) when the extent of hepatocyte death exceeds the liver's regenerative capacity. Although small interfering RNA (siRNA) appears promising in animal models of hepatitis, the approach is limited by drawbacks associated with systemic administration of siRNA. The aim of this study is to develop a hepatocyte-specific delivery system of siRNA for treatment of fulminant hepatitis.
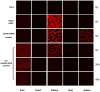
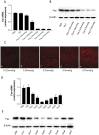
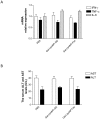
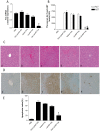
Methodology/principal findings: Galactose-conjugated liposome nano-particles (Gal-LipoNP) bearing siRNA was prepared, and the particle size and zeta potential of Gal-LipoNP/siRNA complexes were measured. The distribution, cytotoxicity and gene silence efficiency were studied in vivo in a concanavalin A (ConA)-induced hepatitis model. C57BL/6 mice were treated with Gal-LipoNP Fas siRNA by i.v. injection 72 h before ConA challenge, and hepatocyte injury was evaluated using serum alanine transferase (ALT) and aspartate transaminase (AST) levels, as well as liver histopathology and TUNEL-positive hepatocytes. The galactose-ligated liposomes were capable of encapsulating >96% siRNA and exhibited a higher stability than naked siRNA in plasma. Hepatocyte-specific targeting was confirmed by in vivo delivery experiment, in which the majority of Gal-LipoNP-siRNA evaded nuclease digestion and accumulated in the liver as soon as 6 h after administration. In vivo gene silencing was significant in the liver after treatment of Gal-Lipo-siRNA. In the ConA-induced hepatitis model, serum levels of ALT and AST were significantly reduced in mice treated with Gal-lipoNP-siRNA as compared with control mice. Additionally, tissue histopathology and apoptosis showed an overall reduction of injury in the Gal-LipoNP siRNA-treated mice.
Conclusions/significance: This study is the first to our knowledge to demonstrate reduction of hepatic injury by liver-specific induction of RNA interference using Gal-LipoNP Fas siRNA, highlighting a novel RNAi-based therapeutic potential in many liver diseases.
Conflict of interest statement
Figures

References
-
- Vaquero J, Blei AT (2003) Etiology and management of fulminant hepatic failure. Curr Gastroenterol Rep 5: 39–47. - PubMed
-
- Sass DA, Shakil AO (2005) Fulminant hepatic failure. Liver Transpl 11: 594–605. - PubMed
-
- Kawakami S, Hashida M (2007) Targeted delivery systems of small interfering RNA by systemic administration. Drug Metab Pharmacokinet 22: 142–151. - PubMed
-
- Akhtar S, Benter I (2007) Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev 59: 164–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

