Identification and characterization of a broadly cross-reactive HIV-1 human monoclonal antibody that binds to both gp120 and gp41
- PMID: 22970187
- PMCID: PMC3438192
- DOI: 10.1371/journal.pone.0044241
Identification and characterization of a broadly cross-reactive HIV-1 human monoclonal antibody that binds to both gp120 and gp41
Abstract
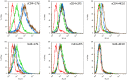
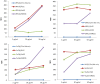
Identification of broadly cross-reactive HIV-1-neutralizing antibodies (bnAbs) may assist vaccine immunogen design. Here we report a novel human monoclonal antibody (mAb), designated m43, which co-targets the gp120 and gp41 subunits of the HIV-1 envelope glycoprotein (Env). M43 bound to recombinant gp140 s from various primary isolates, to membrane-associated Envs on transfected cells and HIV-1 infected cells, as well as to recombinant gp120 s and gp41 fusion intermediate structures containing N-trimer structure, but did not bind to denatured recombinant gp140 s and the CD4 binding site (CD4bs) mutant, gp120 D368R, suggesting that the m43 epitope is conformational and overlaps the CD4bs on gp120 and the N-trimer structure on gp41. M43 neutralized 34% of the HIV-1 primary isolates from different clades and all the SHIVs tested in assays based on infection of peripheral blood mononuclear cells (PBMCs) by replication-competent virus, but was less potent in cell line-based pseudovirus assays. In contrast to CD4, m43 did not induce Env conformational changes upon binding leading to exposure of the coreceptor binding site, enhanced binding of mAbs 2F5 and 4E10 specific for the membrane proximal external region (MPER) of gp41 Envs, or increased gp120 shedding. The overall modest neutralization activity of m43 is likely due to the limited binding of m43 to functional Envs which could be increased by antibody engineering if needed. M43 may represent a new class of bnAbs targeting conformational epitopes overlapping structures on both gp120 and gp41. Its novel epitope and possibly new mechanism(s) of neutralization could helpdesign improved vaccine immunogens and candidate therapeutics.
Conflict of interest statement
Figures




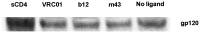
Similar articles
-
HIV type 1 Env precursor cleavage state affects recognition by both neutralizing and nonneutralizing gp41 antibodies.AIDS Res Hum Retroviruses. 2011 Aug;27(8):877-87. doi: 10.1089/AID.2010.0281. Epub 2011 Jan 19. AIDS Res Hum Retroviruses. 2011. PMID: 21158699 Free PMC article.
-
Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens.J Virol. 2008 Jul;82(14):6869-79. doi: 10.1128/JVI.00033-08. Epub 2008 May 14. J Virol. 2008. PMID: 18480433 Free PMC article.
-
Identification and characterization of a new cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody.J Virol. 2004 Sep;78(17):9233-42. doi: 10.1128/JVI.78.17.9233-9242.2004. J Virol. 2004. PMID: 15308718 Free PMC article.
-
Novel approaches for identification of broadly cross-reactive HIV-1 neutralizing human monoclonal antibodies and improvement of their potency.Curr Pharm Des. 2007;13(2):203-12. doi: 10.2174/138161207779313669. Curr Pharm Des. 2007. PMID: 17269928 Review.
-
Neutralizing Antibodies Targeting HIV-1 gp41.Viruses. 2020 Oct 23;12(11):1210. doi: 10.3390/v12111210. Viruses. 2020. PMID: 33114242 Free PMC article. Review.
Cited by
-
Profiling the IgOme: meeting the challenge.FEBS Lett. 2014 Jan 21;588(2):318-25. doi: 10.1016/j.febslet.2013.11.005. Epub 2013 Nov 12. FEBS Lett. 2014. PMID: 24239539 Free PMC article. Review.
-
Analysis of B Cell Receptor Repertoires Reveals Key Signatures of the Systemic B Cell Response after SARS-CoV-2 Infection.J Virol. 2022 Feb 23;96(4):e0160021. doi: 10.1128/JVI.01600-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878902 Free PMC article.
-
Bispecific Anti-HIV Immunoadhesins That Bind Gp120 and Gp41 Have Broad and Potent HIV-Neutralizing Activity.Vaccines (Basel). 2021 Jul 12;9(7):774. doi: 10.3390/vaccines9070774. Vaccines (Basel). 2021. PMID: 34358190 Free PMC article.
-
Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike.Cell Rep. 2014 May 8;7(3):785-95. doi: 10.1016/j.celrep.2014.04.001. Epub 2014 Apr 24. Cell Rep. 2014. PMID: 24767986 Free PMC article.
-
Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers.Immunity. 2014 May 15;40(5):669-80. doi: 10.1016/j.immuni.2014.04.008. Epub 2014 Apr 24. Immunity. 2014. PMID: 24768348 Free PMC article.
References
-
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, et al. (2009) Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458: 636–640. - PubMed
-
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, et al. (1994) Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266: 1024–1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

