The B. subtilis MgtE magnesium transporter can functionally compensate TRPM7-deficiency in vertebrate B-cells
- PMID: 22970223
- PMCID: PMC3435302
- DOI: 10.1371/journal.pone.0044452
The B. subtilis MgtE magnesium transporter can functionally compensate TRPM7-deficiency in vertebrate B-cells
Abstract
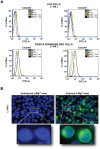
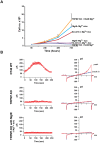
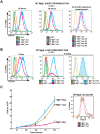
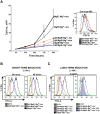
Recent studies have shown that the vertebrate magnesium transporters Solute carrier family 41, members 1 and 2 (SLC41A1, SLC41A2) and Magnesium transporter subtype 1 (MagT1) can endow vertebrate B-cells lacking the ion-channel kinase Transient receptor potential cation channel, subfamily M, member 7 (TRPM7) with a capacity to grow and proliferate. SLC41A1 and SLC41A2 display distant homology to the prokaryotic family of Mg(2+) transporters, MgtE, first characterized in Bacillus subtilis. These sequence similarities prompted us to investigate whether MgtE could potentially compensate for the lack of TRPM7 in the vertebrate TRPM7-deficient DT40 B-cell model system. Here, we report that overexpression of MgtE is able to rescue the growth of TRPM7-KO DT40 B-cells. However, contrary to a previous report that describes regulation of MgtE channel gating by Mg(2+) in a bacterial spheroplast model system, whole cell patch clamp analysis revealed no detectable current development in TRPM7-deficient cells expressing MgtE. In addition, we observed that MgtE expression is strongly downregulated at high magnesium concentrations, similar to what has been described for its vertebrate homolog, SLC41A1. We also show that the N-terminal cytoplasmic domain of MgtE is required for normal MgtE channel function, functionally confirming the predicted importance of this domain in regulation of MgtE-mediated Mg(2+) entry. Overall, our findings show that consistent with its proposed function, Mg(2+) uptake mediated by MgtE is able to restore cell growth and proliferation of TRPM7-deficient cells and supports the concept of functional homology between MgtE and its vertebrate homologs.
Conflict of interest statement
Figures

Similar articles
-
SLC41A2 encodes a plasma-membrane Mg2+ transporter.Biochem J. 2007 Jan 15;401(2):505-13. doi: 10.1042/BJ20060673. Biochem J. 2007. PMID: 16984228 Free PMC article.
-
SLC41A1 Mg(2+) transport is regulated via Mg(2+)-dependent endosomal recycling through its N-terminal cytoplasmic domain.Biochem J. 2011 Oct 1;439(1):129-39. doi: 10.1042/BJ20110807. Biochem J. 2011. PMID: 21696366
-
The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7.FEBS Lett. 2011 Jul 21;585(14):2275-8. doi: 10.1016/j.febslet.2011.05.052. Epub 2011 May 27. FEBS Lett. 2011. PMID: 21627970 Free PMC article.
-
The role of Mg2+ in immune cells.Immunol Res. 2013 Mar;55(1-3):261-9. doi: 10.1007/s12026-012-8371-x. Immunol Res. 2013. PMID: 22990458 Review.
-
SLC41 transporters--molecular identification and functional role.Curr Top Membr. 2014;73:383-410. doi: 10.1016/B978-0-12-800223-0.00011-6. Curr Top Membr. 2014. PMID: 24745990 Review.
Cited by
-
Characterization of a novel MgtE homolog and its structural dynamics in membrane mimetics.Biophys J. 2024 Jul 16;123(14):1968-1983. doi: 10.1016/j.bpj.2023.11.3402. Epub 2023 Dec 1. Biophys J. 2024. PMID: 38042987 Free PMC article.
-
Structural and functional comparison of magnesium transporters throughout evolution.Cell Mol Life Sci. 2022 Jul 12;79(8):418. doi: 10.1007/s00018-022-04442-8. Cell Mol Life Sci. 2022. PMID: 35819535 Free PMC article. Review.
-
Identification of a Mg2+-sensitive ORF in the 5'-leader of TRPM7 magnesium channel mRNA.Nucleic Acids Res. 2014 Nov 10;42(20):12779-88. doi: 10.1093/nar/gku951. Epub 2014 Oct 17. Nucleic Acids Res. 2014. PMID: 25326319 Free PMC article.
-
Gating-related Structural Dynamics of the MgtE Magnesium Channel in Membrane-Mimetics Utilizing Site-Directed Tryptophan Fluorescence.J Mol Biol. 2021 Aug 20;433(17):166691. doi: 10.1016/j.jmb.2020.10.025. Epub 2020 Oct 22. J Mol Biol. 2021. PMID: 33203509 Free PMC article.
-
Transcriptional Profiling Analysis of Bacillus subtilis in Response to High Levels of Fe(3.).Curr Microbiol. 2016 Jun;72(6):653-62. doi: 10.1007/s00284-016-0998-8. Epub 2016 Feb 8. Curr Microbiol. 2016. PMID: 26858131
References
-
- Maguire ME (2006) Magnesium transporters: properties, regulation and structure. Front Biosci 11: 3149–3163. - PubMed
-
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, et al. (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114: 191–200. - PubMed
-
- Mandt T, Song Y, Scharenberg AM, Sahni J (2011) SLC41A1 Mg2+ transport is regulated via Mg2+-dependent endosomal recycling through its N-terminal cytoplasmic domain. Biochem J 439: 129–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

