An exported heat shock protein 40 associates with pathogenesis-related knobs in Plasmodium falciparum infected erythrocytes
- PMID: 22970262
- PMCID: PMC3436795
- DOI: 10.1371/journal.pone.0044605
An exported heat shock protein 40 associates with pathogenesis-related knobs in Plasmodium falciparum infected erythrocytes
Abstract
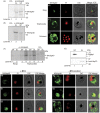
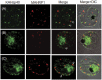
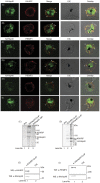
Cell surface structures termed knobs are one of the most important pathogenesis related protein complexes deployed by the malaria parasite Plasmodium falciparum at the surface of the infected erythrocyte. Despite their relevance to the disease, their structure, mechanisms of traffic and their process of assembly remain poorly understood. In this study, we have explored the possible role of a parasite-encoded Hsp40 class of chaperone, namely PFB0090c/PF3D7_0201800 (KAHsp40) in protein trafficking in the infected erythrocyte. We found the gene coding for PF3D7_0201800 to be located in a chromosomal cluster together with knob components KAHRP and PfEMP3. Like the knob components, KAHsp40 too showed the presence of PEXEL motif required for transport to the erythrocyte compartment. Indeed, sub-cellular fractionation and immunofluorescence analysis (IFA) showed KAHsp40 to be exported in the erythrocyte cytoplasm in a stage dependent manner localizing as punctuate spots in the erythrocyte periphery, distinctly from Maurer's cleft, in structures which could be the reminiscent of knobs. Double IFA analysis revealed co-localization of PF3D7_0201800 with the markers of knobs (KAHRP, PfEMP1 and PfEMP3) and components of the PEXEL translocon (Hsp101, PTEX150). KAHsp40 was also found to be in a complex with KAHRP, PfEMP3 and Hsp101 as confirmed by co-immunoprecipitation assay. Our results suggest potential involvement of a parasite encoded Hsp40 in chaperoning knob assembly in the erythrocyte compartment.
Conflict of interest statement
Figures


References
-
- Marti M, Good RT, Rug M, Knuepfer E, Cowman AF (2004) Targeting malaria virulence and remodelling proteins to the host erythrocyte. Science 306: 1930–1933. - PubMed
-
- Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, et al. (2004) A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306: 1934–1937. - PubMed
-
- Lanzer M, Wickert H, Krohne G, Vincensini L, Braun Breton C (2006) Maurer’s clefts: a novel multi-functional organelle in the cytoplasm of Plasmodium falciparum- infected erythrocytes. Int J Parasitol 36 23–36. - PubMed
-
- Tilley L, Sougrat R, Lithgow T, Hanssen E (2008) The twists and turns of Maurer’s cleft trafficking in P. falciparum- infected erythrocytes. Traffic 9 187–197. - PubMed
-
- Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, et al. (1995) The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82 89–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

