Development of multiplexed bead-based immunoassays for the detection of early stage ovarian cancer using a combination of serum biomarkers
- PMID: 22970327
- PMCID: PMC3438175
- DOI: 10.1371/journal.pone.0044960
Development of multiplexed bead-based immunoassays for the detection of early stage ovarian cancer using a combination of serum biomarkers
Abstract
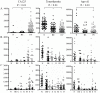
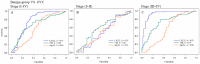
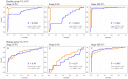
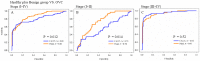
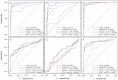
CA125 as a biomarker of ovarian cancer is ineffective for the general population. The aim of this study was to evaluate multiplexed bead-based immunoassay of multiple ovarian cancer-associated biomarkers such as transthyretin and apolipoprotein A1, together with CA125, to improve the identification and evaluation of prognosis of ovarian cancer. We measured the serum levels of CA125, transthyretin, and apolipoprotein A1 from the serum of 61 healthy individuals, 84 patients with benign ovarian disease, and 118 patients with ovarian cancer using a multiplex liquid assay system, Luminex 100. The results were then analyzed according to healthy and/or benign versus ovarian cancer subjects. When CA125 was combined with the other biomarkers, the overall sensitivity and specificity were significantly improved in the ROC curve, which showed 95% and 97% sensitivity and specificity, respectively. At 95% specificity for all stages the sensitivity increased to 95.5% compared to 67% for CA125 alone. For stage I+II, the sensitivity increased from 30% for CA125 alone to 93.9%. For stage III+IV, the corresponding values were 96.5% and 91.6%, respectively. Also, the three biomarkers were sufficient for maximum separation between noncancer (healthy plus benign group) and stage I+II or all stages (I-IV) of disease. The new combination of transthyretin, and apolipoprotein A1 with CA125 improved both the sensitivity and the specificity of ovarian cancer diagnosis compared with those of individual biomarkers. These findings suggest the benefit of the combination of these markers for the diagnosis of ovarian cancer.
Conflict of interest statement
Figures
Similar articles
-
Multiplexed bead-based immunoassay of four serum biomarkers for diagnosis of ovarian cancer.Oncol Rep. 2012 Aug;28(2):585-91. doi: 10.3892/or.2012.1829. Epub 2012 May 22. Oncol Rep. 2012. PMID: 22641176
-
Proteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein III enhance the sensitivity of CA125 for detecting early stage epithelial ovarian cancer.Gynecol Oncol. 2011 Sep;122(3):548-53. doi: 10.1016/j.ygyno.2011.06.002. Epub 2011 Jun 25. Gynecol Oncol. 2011. PMID: 21708402 Free PMC article.
-
A multiplex biomarker assay improves the diagnostic performance of HE4 and CA125 in ovarian tumor patients.PLoS One. 2020 Oct 19;15(10):e0240418. doi: 10.1371/journal.pone.0240418. eCollection 2020. PLoS One. 2020. PMID: 33075095 Free PMC article.
-
New tumor markers: CA125 and beyond.Int J Gynecol Cancer. 2005 Nov-Dec;15 Suppl 3:274-81. doi: 10.1111/j.1525-1438.2005.00441.x. Int J Gynecol Cancer. 2005. PMID: 16343244 Review.
-
Screening for Ovarian Cancer in the General Population: State of Art and Perspectives of Clinical Research.Anticancer Res. 2022 Sep;42(9):4207-4216. doi: 10.21873/anticanres.15921. Anticancer Res. 2022. PMID: 36039417 Review.
Cited by
-
Review of biomarker systems as an alternative for early diagnosis of ovarian carcinoma.Clin Transl Oncol. 2021 Oct;23(10):1967-1978. doi: 10.1007/s12094-021-02604-x. Epub 2021 Apr 11. Clin Transl Oncol. 2021. PMID: 33840014 Review.
-
An Improved Prediction Model for Ovarian Cancer Using Urinary Biomarkers and a Novel Validation Strategy.Int J Mol Sci. 2019 Oct 5;20(19):4938. doi: 10.3390/ijms20194938. Int J Mol Sci. 2019. PMID: 31590408 Free PMC article.
-
WFDC Protein: A Promising Diagnosis Biomarker of Ovarian Cancer.J Cancer. 2021 Jul 6;12(18):5404-5412. doi: 10.7150/jca.57880. eCollection 2021. J Cancer. 2021. PMID: 34405003 Free PMC article. Review.
-
Serum Apolipoprotein A-I Combined With C-Reactive Protein Serves As A Novel Prognostic Stratification System For Colorectal Cancer.Cancer Manag Res. 2019 Oct 30;11:9265-9276. doi: 10.2147/CMAR.S215599. eCollection 2019. Cancer Manag Res. 2019. PMID: 31802946 Free PMC article.
-
HDL in Endocrine Carcinomas: Biomarker, Drug Carrier, and Potential Therapeutic.Front Endocrinol (Lausanne). 2018 Nov 30;9:715. doi: 10.3389/fendo.2018.00715. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30555417 Free PMC article. Review.
References
-
- Morice P, Brehier-Ollive D, Rey A, Atallah D, Lhomme C, et al. (2003) Results of interval debulking surgery in advanced stage ovarian cancer: an exposed-non-exposed study. Ann Oncol 14: 74–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

