Glyoxylate carboligase: a unique thiamin diphosphate-dependent enzyme that can cycle between the 4'-aminopyrimidinium and 1',4'-iminopyrimidine tautomeric forms in the absence of the conserved glutamate
- PMID: 22970650
- PMCID: PMC3493109
- DOI: 10.1021/bi300893v
Glyoxylate carboligase: a unique thiamin diphosphate-dependent enzyme that can cycle between the 4'-aminopyrimidinium and 1',4'-iminopyrimidine tautomeric forms in the absence of the conserved glutamate
Abstract
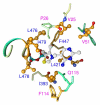
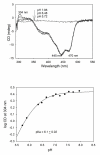
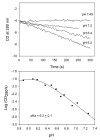
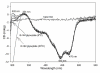
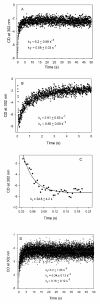
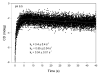
Glyoxylate carboligase (GCL) is a thiamin diphosphate (ThDP)-dependent enzyme, which catalyzes the decarboxylation of glyoxylate and ligation to a second molecule of glyoxylate to form tartronate semialdehyde (TSA). This enzyme is unique among ThDP enzymes in that it lacks a conserved glutamate near the N1' atom of ThDP (replaced by Val51) or any other potential acid-base side chains near ThDP. The V51D substitution shifts the pH optimum to 6.0-6.2 (pK(a) of 6.2) for TSA formation from pH 7.0-7.7 in wild-type GCL. This pK(a) is similar to the pK(a) of 6.1 for the 1',4'-iminopyrimidine (IP)-4'-aminopyrimidinium (APH(+)) protonic equilibrium, suggesting that the same groups control both ThDP protonation and TSA formation. The key covalent ThDP-bound intermediates were identified on V51D GCL by a combination of steady-state and stopped-flow circular dichroism methods, yielding rate constants for their formation and decomposition. It was demonstrated that active center variants with substitution at I393 could synthesize (S)-acetolactate from pyruvate solely, and acetylglycolate derived from pyruvate as the acetyl donor and glyoxylate as the acceptor, implying that this substitutent favored pyruvate as the donor in carboligase reactions. Consistent with these observations, the I393A GLC variants could stabilize the predecarboxylation intermediate analogues derived from acetylphosphinate, propionylphosphinate, and methyl acetylphosphonate in their IP tautomeric forms notwithstanding the absence of the conserved glutamate. The role of the residue at the position occupied typically by the conserved Glu controls the pH dependence of kinetic parameters, while the entire reaction sequence could be catalyzed by ThDP itself, once the APH(+) form is accessible.
Figures





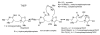
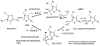
Similar articles
-
Bifunctionality of the thiamin diphosphate cofactor: assignment of tautomeric/ionization states of the 4'-aminopyrimidine ring when various intermediates occupy the active sites during the catalysis of yeast pyruvate decarboxylase.J Am Chem Soc. 2012 Feb 29;134(8):3873-85. doi: 10.1021/ja211139c. Epub 2012 Feb 17. J Am Chem Soc. 2012. PMID: 22300533 Free PMC article.
-
Double duty for a conserved glutamate in pyruvate decarboxylase: evidence of the participation in stereoelectronically controlled decarboxylation and in protonation of the nascent carbanion/enamine intermediate.Biochemistry. 2010 Sep 21;49(37):8197-212. doi: 10.1021/bi100828r. Biochemistry. 2010. PMID: 20715795 Free PMC article.
-
Glutamate 636 of the Escherichia coli pyruvate dehydrogenase-E1 participates in active center communication and behaves as an engineered acetolactate synthase with unusual stereoselectivity.J Biol Chem. 2005 Jun 3;280(22):21473-82. doi: 10.1074/jbc.M502691200. Epub 2005 Mar 31. J Biol Chem. 2005. PMID: 15802265
-
Reaction mechanisms of thiamin diphosphate enzymes: new insights into the role of a conserved glutamate residue.FEBS J. 2009 May;276(9):2447-53. doi: 10.1111/j.1742-4658.2009.06965.x. Epub 2009 Mar 16. FEBS J. 2009. PMID: 19476486 Review.
-
Progress in the experimental observation of thiamin diphosphate-bound intermediates on enzymes and mechanistic information derived from these observations.Bioorg Chem. 2014 Dec;57:251-262. doi: 10.1016/j.bioorg.2014.08.002. Epub 2014 Sep 3. Bioorg Chem. 2014. PMID: 25228115 Review.
Cited by
-
Identification of charge transfer transitions related to thiamin-bound intermediates on enzymes provides a plethora of signatures useful in mechanistic studies.Biochemistry. 2014 Apr 8;53(13):2145-52. doi: 10.1021/bi4015743. Epub 2014 Mar 26. Biochemistry. 2014. PMID: 24628377 Free PMC article.
-
Diversity of ThDP-Dependent Enzymes Forming Chiral Tertiary Alcohols.Chembiochem. 2025 Jul 11;26(13):e202500200. doi: 10.1002/cbic.202500200. Epub 2025 May 27. Chembiochem. 2025. PMID: 40228089 Free PMC article. Review.
-
Human 2-Oxoglutarate Dehydrogenase and 2-Oxoadipate Dehydrogenase Both Generate Superoxide/H2O2 in a Side Reaction and Each Could Contribute to Oxidative Stress in Mitochondria.Neurochem Res. 2019 Oct;44(10):2325-2335. doi: 10.1007/s11064-019-02765-w. Epub 2019 Mar 7. Neurochem Res. 2019. PMID: 30847859 Review.
-
Evidence of Diradicals Involved in the Yeast Transketolase Catalyzed Keto-Transferring Reactions.Chembiochem. 2018 Nov 16;19(22):2395-2402. doi: 10.1002/cbic.201800378. Epub 2018 Oct 18. Chembiochem. 2018. PMID: 30155962 Free PMC article.
-
DNA microarray of global transcription factor mutant reveals membrane-related proteins involved in n-butanol tolerance in Escherichia coli.Biotechnol Biofuels. 2016 Jun 1;9:114. doi: 10.1186/s13068-016-0527-9. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27252779 Free PMC article.
References
-
- Chang YY, Wang AY, Cronan JE., Jr Molecular cloning, DNA sequencing, and biochemical analyses of Escherichia coli glyoxylate carboligase. An enzyme of the acetohydroxy acid synthase - pyruvate oxidase family. J. Biol. Chem. 1993;268:3911–3919. - PubMed
-
- Gupta NK, Vennesland B. Glyoxylate Carboxylase of Escherichia coli: a Flavoprotein. J. Biol. Chem. 1964;239:3787–3789. - PubMed
-
- Gupta N, Vennesland B. Glyoxylate carboligase of Escherichia coli: some properties of the enzyme. Arch. Biochem. Biophys. 1966;113:255–264. - PubMed
-
- Cromatie TH, Walsh CT. Escherichia coli glyoxalate carboligase. Properties and reconstitution with 5-deazaFAD and 1,5-dihydrodeazaFADH2. J. Biol. Chem. 1976;251:329–333. - PubMed
-
- Kaplun A, Binshtein E, Vyazmensky M, Steinmetz A, Barak Z, Chipman DM, Tittmann K, Shaanan B. Glyoxylate carboligase lacks the canonical active site glutamate of thiamin-dependent enzymes. Nat. Chem. Biol. 2008;4:113–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

