Molecular mechanism for the preferential exclusion of TMAO from protein surfaces
- PMID: 22970901
- PMCID: PMC3534762
- DOI: 10.1021/jp304298c
Molecular mechanism for the preferential exclusion of TMAO from protein surfaces
Abstract
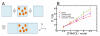
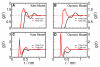
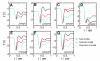
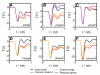
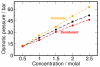
Trimethylamine N-oxide (TMAO) is a naturally occurring protecting osmolyte that stabilizes the folded state of proteins and also counteracts the destabilizing effect of urea on protein stability. Experimentally, it has been inferred that TMAO is preferentially excluded from the vicinity of protein surfaces. Here, we combine computer modeling and experimental measurements to gain an understanding of the mechanism of the protecting effect of TMAO on proteins. We have developed an all-atom molecular model for TMAO that captures the exclusion of TMAO from model compounds and protein surfaces, as a consequence of incorporating realistic TMAO-water interactions through osmotic pressure measurements. Osmotic pressure measurements also suggest no significant attraction between urea and TMAO molecules in solution. To obtain an accurate potential for molecular simulations of protein stability in TMAO solutions, we have explored different ways of parametrizing the protein/osmolyte and osmolyte/osmolyte interactions by scaling charges and the strength of Lennard-Jones interactions and carried out equilibrium folding experiments of Trp-cage miniprotein in the presence of TMAO to guide the parametrization. Our calculations suggest a general principle for preferential interaction behavior of cosolvents with protein surfaces--preferentially excluded osmolytes have repulsive self-interaction given by osmotic coefficient φ > 1, while denaturants, in addition to having attractive interactions with the proteins, have favorable self-interaction given by osmotic coefficient φ < 1, to enable preferential accumulation in the vicinity of proteins.
Figures



Similar articles
-
Cosolvent effects on protein stability.Annu Rev Phys Chem. 2013;64:273-93. doi: 10.1146/annurev-physchem-040412-110156. Epub 2013 Jan 4. Annu Rev Phys Chem. 2013. PMID: 23298246 Review.
-
Trimethylamine N-oxide stabilizes proteins via a distinct mechanism compared with betaine and glycine.Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):2479-2484. doi: 10.1073/pnas.1614609114. Epub 2017 Feb 22. Proc Natl Acad Sci U S A. 2017. PMID: 28228526 Free PMC article.
-
Osmolyte trimethylamine-N-oxide does not affect the strength of hydrophobic interactions: origin of osmolyte compatibility.Biophys J. 2005 Aug;89(2):858-66. doi: 10.1529/biophysj.104.056671. Epub 2005 May 13. Biophys J. 2005. PMID: 15894642 Free PMC article.
-
Interactions of S-peptide analogue in aqueous urea and trimethylamine-N-oxide solutions: a molecular dynamics simulation study.J Chem Phys. 2013 Jul 21;139(3):034504. doi: 10.1063/1.4813502. J Chem Phys. 2013. PMID: 23883044
-
Protein Stability in TMAO and Mixed Urea-TMAO Solutions.J Phys Chem B. 2020 Jul 23;124(29):6181-6197. doi: 10.1021/acs.jpcb.0c04357. Epub 2020 Jun 25. J Phys Chem B. 2020. PMID: 32495623 Review.
Cited by
-
Trimethylamine N-oxide: role in cell senescence and age-related diseases.Eur J Nutr. 2023 Mar;62(2):525-541. doi: 10.1007/s00394-022-03011-w. Epub 2022 Oct 11. Eur J Nutr. 2023. PMID: 36219234 Review.
-
Distinctive solvation patterns make renal osmolytes diverse.Biophys J. 2013 Nov 5;105(9):2166-74. doi: 10.1016/j.bpj.2013.09.019. Biophys J. 2013. PMID: 24209862 Free PMC article.
-
Restoration of structural stability and ligand binding after removal of the conserved disulfide bond in tear lipocalin.Biochem Biophys Res Commun. 2014 Oct 3;452(4):1004-8. doi: 10.1016/j.bbrc.2014.09.029. Epub 2014 Sep 16. Biochem Biophys Res Commun. 2014. PMID: 25223802 Free PMC article.
-
Solvation free energy of the peptide group: its model dependence and implications for the additive-transfer free-energy model of protein stability.Biophys J. 2013 Sep 17;105(6):1482-90. doi: 10.1016/j.bpj.2013.08.011. Biophys J. 2013. PMID: 24048000 Free PMC article.
-
Diffusiophoresis of Macromolecules within the Framework of Multicomponent Diffusion.Molecules. 2024 Mar 19;29(6):1367. doi: 10.3390/molecules29061367. Molecules. 2024. PMID: 38543003 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

