Dissecting the role of human embryonic stem cell-derived mesenchymal cells in human umbilical vein endothelial cell network stabilization in three-dimensional environments
- PMID: 22971005
- PMCID: PMC3530951
- DOI: 10.1089/ten.tea.2011.0408
Dissecting the role of human embryonic stem cell-derived mesenchymal cells in human umbilical vein endothelial cell network stabilization in three-dimensional environments
Abstract
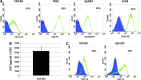
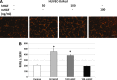
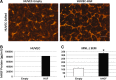
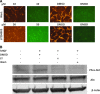
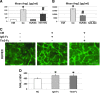
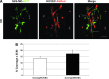
The microvasculature is principally composed of two cell types: endothelium and mural support cells. Multiple sources are available for human endothelial cells (ECs) but sources for human microvascular mural cells (MCs) are limited. We derived multipotent mesenchymal progenitor cells from human embryonic stem cells (hES-MC) that can function as an MC and stabilize human EC networks in three-dimensional (3D) collagen-fibronectin culture by paracrine mechanisms. Here, we have investigated the basis for hES-MC-mediated stabilization and identified the pleiotropic growth factor hepatocyte growth factor/scatter factor (HGF/SF) as a putative hES-MC-derived regulator of EC network stabilization in 3D in vitro culture. Pharmacological inhibition of the HGF receptor (Met) (1 μm SU11274) inhibits EC network formation in the presence of hES-MC. hES-MC produce and release HGF while human umbilical vein endothelial cells (HUVEC) do not. When HUVEC are cultured alone the networks collapse, but in the presence of recombinant human HGF or conditioned media from human HGF-transduced cells significantly more networks persist. In addition, HUVEC transduced to constitutively express human HGF also form stable networks by autocrine mechanisms. By enzyme-linked immunosorbent assay, the coculture media were enriched in both angiopoietin-1 (Ang1) and angiopoietin-2 (Ang2), but at significantly different levels (Ang1=159±15 pg/mL vs. Ang2=30,867±2685 pg/mL) contributed by hES-MC and HUVEC, respectively. Although the coculture cells formed stabile network architectures, their morphology suggests the assembly of an immature plexus. When HUVEC and hES-MC were implanted subcutaneously in immune compromised Rag1 mice, hES-MC increased their contact with HUVEC along the axis of the vessel. This data suggests that HUVEC and hES-MC form an immature plexus mediated in part by HGF and angiopoietins that is capable of maturation under the correct environmental conditions (e.g., in vivo). Therefore, hES-MC can function as microvascular MCs and may be a useful cell source for testing EC-MC interactions.
Figures


References
-
- D'Amore P.A. Capillary growth: a two-cell system. Semin Cancer Biol. 1992;3:49. - PubMed
-
- Risau W. Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73. - PubMed
-
- Sabin F.R. Studies on the origin of blood-vessels and of red blood-corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Anat Rec. 1917;13:199.
-
- Furuta C. Ema H. Takayanagi S. Ogaeri T. Okamura D. Matsui Y., et al. Discordant developmental waves of angioblasts and hemangioblasts in the early gastrulating mouse embryo. Development. 2006;133:2771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

