Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes
- PMID: 22971339
- PMCID: PMC3480645
- DOI: 10.1016/j.jmb.2012.09.001
Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes
Abstract
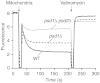
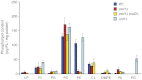
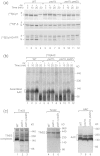
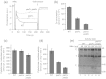
The mitochondrial inner membrane contains two non-bilayer-forming phospholipids, phosphatidylethanolamine (PE) and cardiolipin (CL). Lack of CL leads to destabilization of respiratory chain supercomplexes, a reduced activity of cytochrome c oxidase, and a reduced inner membrane potential Δψ. Although PE is more abundant than CL in the mitochondrial inner membrane, its role in biogenesis and assembly of inner membrane complexes is unknown. We report that similar to the lack of CL, PE depletion resulted in a decrease of Δψ and thus in an impaired import of preproteins into and across the inner membrane. The respiratory capacity and in particular the activity of cytochrome c oxidase were impaired in PE-depleted mitochondria, leading to the decrease of Δψ. In contrast to depletion of CL, depletion of PE did not destabilize respiratory chain supercomplexes but favored the formation of larger supercomplexes (megacomplexes) between the cytochrome bc(1) complex and the cytochrome c oxidase. We conclude that both PE and CL are required for a full activity of the mitochondrial respiratory chain and the efficient generation of the inner membrane potential. The mechanisms, however, are different since these non-bilayer-forming phospholipids exert opposite effects on the stability of respiratory chain supercomplexes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
-
- Schuiki I., Daum G. Phosphatidylserine decarboxylase, key enzymes of lipid metabolism. IUBMB Life. 2009;61:151–162. - PubMed
-
- Storey M.K., Clay K.L., Kutateladze T., Murphy R.C., Overduin M., Voelker D.R. Phosphatidylethanolamine has an essential role in Saccharomyces cerevisiae that is independent of its ability to form hexagonal phase structures. J. Biol. Chem. 2001;276:48539–48548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

