Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors
- PMID: 22971343
- PMCID: PMC3685485
- DOI: 10.1158/0008-5472.CAN-12-1335
Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors
Abstract
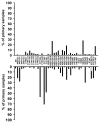
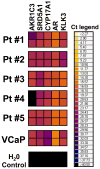
Androgen receptor (AR) signaling persists in castration-resistant prostate carcinomas (CRPC), because of several mechanisms that include increased AR expression and intratumoral androgen metabolism. We investigated the mechanisms underlying aberrant expression of transcripts involved in androgen metabolism in CRPC. We compared gene expression profiles and DNA copy number alteration (CNA) data from 29 normal prostate tissue samples, 127 primary prostate carcinomas (PCa), and 19 metastatic PCas. Steroidogenic enzyme transcripts were evaluated by quantitative reverse transcriptase PCR in PCa cell lines and circulating tumor cells (CTC) from CRPC patients. Metastatic PCas expressed higher transcript levels for AR and several steroidogenic enzymes, including SRD5A1, SRD5A3, and AKR1C3, whereas expression of SRD5A2, CYP3A4, CYP3A5, and CYP3A7 was decreased. This aberrant expression was rarely associated with CNAs. Instead, our data suggest distinct patterns of coordinated aberrant enzyme expression. Inhibition of AR activity by itself stimulated AKR1C3 expression. The aberrant expression of the steroidogenic enzyme transcripts was detected in CTCs from CRPC patients. In conclusion, our findings identify substantial interpatient heterogeneity and distinct patterns of dysregulated expression of enzymes involved in intratumoral androgen metabolism in PCa. These steroidogenic enzymes represent targets for complete suppression of systemic and intratumoral androgen levels, an objective that is supported by the clinical efficacy of the CYP17 inhibitor abiraterone. A comprehensive AR axis-targeting approach via simultaneous, frontline enzymatic blockade, and/or transcriptional repression of several steroidogenic enzymes, in combination with GnRH analogs and potent antiandrogens, would represent a powerful future strategy for PCa management.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





Similar articles
-
Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer.Cancer Res. 2010 Feb 1;70(3):1256-64. doi: 10.1158/0008-5472.CAN-09-2092. Epub 2010 Jan 19. Cancer Res. 2010. PMID: 20086173
-
Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth.Clin Cancer Res. 2013 Oct 15;19(20):5613-25. doi: 10.1158/1078-0432.CCR-13-1151. Epub 2013 Aug 30. Clin Cancer Res. 2013. PMID: 23995860
-
ERG/AKR1C3/AR Constitutes a Feed-Forward Loop for AR Signaling in Prostate Cancer Cells.Clin Cancer Res. 2015 Jun 1;21(11):2569-79. doi: 10.1158/1078-0432.CCR-14-2352. Epub 2015 Mar 9. Clin Cancer Res. 2015. PMID: 25754347 Free PMC article.
-
AKR1C3 as a target in castrate resistant prostate cancer.J Steroid Biochem Mol Biol. 2013 Sep;137:136-49. doi: 10.1016/j.jsbmb.2013.05.012. Epub 2013 Jun 6. J Steroid Biochem Mol Biol. 2013. PMID: 23748150 Free PMC article. Review.
-
Key targets of hormonal treatment of prostate cancer. Part 1: the androgen receptor and steroidogenic pathways.BJU Int. 2009 Aug;104(4):438-48. doi: 10.1111/j.1464-410X.2009.08695.x. Epub 2009 Jun 24. BJU Int. 2009. PMID: 19558559 Review.
Cited by
-
Aldo-keto reductases: Role in cancer development and theranostics.Oncol Res. 2024 Jul 17;32(8):1287-1308. doi: 10.32604/or.2024.049918. eCollection 2024. Oncol Res. 2024. PMID: 39055885 Free PMC article. Review.
-
Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover.Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):6997-7002. doi: 10.1073/pnas.1304502110. Epub 2013 Apr 4. Proc Natl Acad Sci U S A. 2013. PMID: 23559371 Free PMC article.
-
Inhibition of AKR1C3 Activation Overcomes Resistance to Abiraterone in Advanced Prostate Cancer.Mol Cancer Ther. 2017 Jan;16(1):35-44. doi: 10.1158/1535-7163.MCT-16-0186. Epub 2016 Oct 28. Mol Cancer Ther. 2017. PMID: 27794047 Free PMC article.
-
Synergistic action of image-guided radiotherapy and androgen deprivation therapy.Nat Rev Urol. 2015 Apr;12(4):193-204. doi: 10.1038/nrurol.2015.50. Epub 2015 Mar 24. Nat Rev Urol. 2015. PMID: 25800395 Review.
-
The role of adrenal derived androgens in castration resistant prostate cancer.J Steroid Biochem Mol Biol. 2020 Mar;197:105506. doi: 10.1016/j.jsbmb.2019.105506. Epub 2019 Oct 28. J Steroid Biochem Mol Biol. 2020. PMID: 31672619 Free PMC article. Review.
References
-
- Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

