Genetic visualization of notch signaling in mammalian neurogenesis
- PMID: 22971775
- PMCID: PMC3663255
- DOI: 10.1007/s00018-012-1151-x
Genetic visualization of notch signaling in mammalian neurogenesis
Abstract
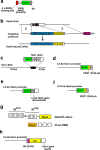
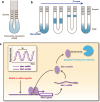
Notch signaling plays crucial roles in fate determination and the differentiation of neural stem cells in embryonic and adult brains. It is now clear that the notch pathway is under more complex and dynamic regulation than previously thought. To understand the functional details of notch signaling more precisely, it is important to reveal when, where, and how notch signaling is dynamically communicated between cells, for which the visualization of notch signaling is essential. In this review, we introduce recent technical advances in the visualization of notch signaling during neural development and in the adult brain, and we discuss the physiological significance of dynamic regulation of notch signaling.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

