Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review
- PMID: 22972056
- PMCID: PMC7061650
- DOI: 10.1002/14651858.CD001901.pub2
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review
Abstract
Background: Epilepsy is an important neurological condition and drug resistance in epilepsy is particularly common in individuals with focal seizures. In this review, we summarise the current evidence regarding a new antiepileptic drug, levetiracetam, when used as add-on treatment for controlling drug-resistant focal epilepsy. This is an update to a Cochrane Review that was originally published in 2001.
Objectives: To evaluate the effectiveness of levetiracetam, added on to usual care, in treating drug-resistant focal epilepsy.
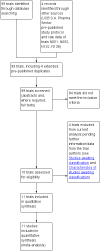
Search methods: We searched the Cochrane Epilepsy Group's Specialized Register (August 2012), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 7, 2012), and MEDLINE (1946 to August week 1, 2012). We also contacted the manufacturers of levetiracetam and researchers in the field to seek any ongoing or unpublished trials.
Selection criteria: Randomised, placebo-controlled trials of add-on levetiracetam treatment in people with drug-resistant focal epilepsy.
Data collection and analysis: Two review authors independently selected trials for inclusion, assessed trials for bias, extracted data, and evaluated the overall quality of evidence. Outcomes investigated included 50% or greater reduction in focal seizure frequency (response); less than 50% reduction in focal seizure frequency (non-response); treatment withdrawal; adverse effects (including a specific analysis of changes in behaviour); cognitive effects and quality of life (QoL). Risk ratios (RR) with 95% confidence intervals (CIs) were used as measures of effect (99% CIs for adverse effects). Primary analyses were Intention-to-Treat (ITT). Dose response and inter-trial heterogeneity were evaluated in regression models.
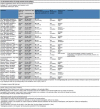
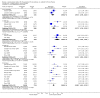
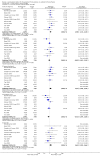
Main results: Eleven trials (1861 participants) were included. They predominantly possessed low risks of bias. Participants were adults in nine trials (1565 participants) and children in the remaining two trials (296 participants). The dose of levetiracetam tested was 1000 to 4000 mg/day in adults, and 60 mg/kg/day in children. Treatment ranged from 12 to 24 weeks. For the 50% or greater reduction in focal seizure frequency outcome, the RR was significantly in favour of levetiracetam at all doses. The naive estimates, ignoring dose, showed children (52% responded) as better responders than adults (39% responded) on levetiracetam. 25% of children and 16% of adults responded to placebo. The Number Needed to Treat for an additional beneficial outcome for children and adults was four (95% CI three to seven) and five (95% CI four to six), respectively. The significant levels of statistical heterogeneity between trials on adults precluded valid provision of an overall RR (ignoring dose). Results for the two trials that tested levetiracetam 2000 mg on adults were sufficiently similar to be combined to give an RR for 50% or greater reduction in focal seizure frequency of 4.91 (95% CI 2.75 to 8.77), with an RR of 0.68 (95% CI 0.60 to 0.77) for non-response. At this dose, 37% and 8% of adults were responders in the levetiracetam and placebo groups, respectively. Regression analysis demonstrated that much of the heterogeneity between adult trials was likely to be explained by different doses of levetiracetam tested and different years of trial publication. There was no evidence of statistical heterogeneity between trials on children. For these trials, the RR for 50% or greater reduction in focal seizure frequency was 1.91 (95% CI 1.38 to 2.63), with an RR of 0.68 (95% CI 0.56 to 0.81) for non-response. 27% of children responded. Participants were not significantly more likely to have levetiracetam withdrawn (RR 0.98; 95% CI 0.73 to 1.32 and RR 0.80; 95% CI 0.43 to 1.46 for adults and children, respectively). For adults, somnolence (RR 1.51; 99% CI 1.06 to 2.17) and infection (RR 1.76; 99% CI 1.03 to 3.02) were significantly associated with levetiracetam. Accidental injury was significantly associated with placebo (RR 0.60; 99% CI 0.39 to 0.92). No individual adverse effect was significantly associated with levetiracetam in children. Changes in behaviour were negligible in adults (1% affected; RR 1.79; 99% CI 0.59 to 5.41) but significant in children (23% affected; RR 1.90; 99% CI 1.16 to 3.11). Cognitive effect and QoL outcomes suggested that levetiracetam had a positive effect on cognition and some aspects of QoL in adults. In children, levetiracetam did not appear to alter cognitive function but there was evidence of worsening in certain aspects of child behaviour. The overall quality of evidence used was high.
Authors' conclusions: This update adds seven more trials to the original review, which contained four trials. At every dose analysed, levetiracetam significantly reduced focal seizure frequency relative to placebo. This indicates that levetiracetam can significantly reduce focal seizure frequency when it is used as an add-on treatment for both adults and children with drug-resistant focal epilepsy. As there was evidence of significant levels of statistical heterogeneity within this positive effect it is difficult to be precise about the relative magnitude of the effect. At a dose of 2000 mg, levetiracetam may be expected to be 3.9 times more effective than placebo; with 30% of adults being responders at this dose. At a dose of 60 mg/kg/day, levetiracetam may be expected to be 0.9 times more effective than placebo; with 25% of children being responders at this dose. When dose was ignored, children were better responders than adults by around 4% to 13%. The results grossly suggest that one child or adult may respond to levetiracetam for every four or five children or adults, respectively, that have received levetiracetam rather than placebo. The drug seems to be well tolerated in both adults and children although non-specific changes in behaviour may be experienced in as high as 20% of children. This aspect of the adverse-effect profile of levetiracetam was analysed crudely and requires further investigation and validation. It seems reasonable to continue the use of levetiracetam in both adults and children with drug-resistant focal epilepsy. The results cannot be used to confirm longer-term or monotherapy effects of levetiracetam or its effects on generalised seizures. The conclusions are largely unchanged from those in the original review. The most significant contribution of this update is the addition of paediatric data into the analysis.
Conflict of interest statement
Pete Dixon is funded as part of a research programme (RP‐PG‐0606‐1062) that receives financial support from the National Institute for Health Research (NIHR) Programme Grants for Applied Research (PGfAR) funding scheme. The original review (Chaisewikul 2001) was funded by the Health Technology Assessment (HTA) programme. The views and opinions expressed within this article do not necessarily reflect those of the National Health Service (NHS), the HTA, or the Department of Health. The researchers are independent from the funders.
Figures








Update of
-
Levetiracetam add-on for drug-resistant localization related (partial) epilepsy.Cochrane Database Syst Rev. 2001;(1):CD001901. doi: 10.1002/14651858.CD001901. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2012 Sep 12;(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. PMID: 11279737 Updated.
References
References to studies included in this review
Ben‐Menachem 2000 {unpublished data only}
-
- Ben‐Menachem E, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory seizures: a multicenter, double‐blind, responder‐selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia 2000;41(10):1276‐83. - PubMed
-
- UCB. Evaluation of the efficacy and tolerability of UCB L059 (1500 mg b.i.d., 500 mg tablets) monotherapy in epileptic patients with complex partial onset seizures, having experienced improved seizure control under add‐on treatment: a 60‐week (maximum), double‐blind, multicenter, responder‐selected trial (phase III study, placebo comparator). UCB S.A. Pharma Sector.
Betts 2000 {published and unpublished data}
-
- Betts T, Waegemans T, Crawford P. A multicentre, double‐blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure 2000;9:80‐7. - PubMed
-
- UCB. A 24‐week multicentered, double‐blind, parallel group, add‐on study to compare the efficacy and the tolerability of 2 oral daily doses of UCB L059 (2000 mg and 4000 mg tablets) with placebo in patients with refractory epilepsy, followed by a 24‐week open‐label active treatment. UCB S.A. Pharma Sector.
Cereghino & Cramer 2000 {published and unpublished data}
-
- Cereghino JJ, Biton V, Abou‐Khalil B, Dreifuss F, Gauer LJ, Leppik I, and the United States Levetiracetam Study Group. Levetiracetam for partial seizures: results of a double‐blind, randomized clinical trial. Neurology 2000;55:236‐42. - PubMed
-
- Cramer JA, Arrigo C, Hammee G, Gauer LJ, Cereghino JJ. Effect of levetiracetam on epilepsy‐related quality of life: N132 study group. Epilepsia 2000;41(7):868‐74. - PubMed
-
- UCB. Evaluation of the efficacy and tolerability of UCB L059 (500 and 1500 mg bid, tablets) add‐on treatment in epileptic patients with partial onset seizures: a 38‐week, double‐blind, placebo‐controlled, parallel‐group multicenter trial. UCB S.A. Pharma Sector.
Glauser 2006 {published data only}
-
- Glauser TA, Ayala R, Elterman RD, Mitchell WG, Orman CB, Gauer LJ, et al. Double‐blind placebo‐controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology 2006;66(11):1654‐60. - PubMed
Levisohn 2009 & Loge 2010 {published data only (unpublished sought but not used)}
-
- Levisohn PM, Mintz M, Hunter SJ, Yang H, Jones J. Neurocognitive effects of adjunctive levetiracetam in children with partial‐onset seizures: a randomized, double‐blind, placebo‐controlled, noninferiority trial. Epilepsia 2009;50(11):2377‐89. - PubMed
-
- Loge C, Hunter SJ, Schiemann J, Yang HC. Assessment of behavioral and emotional functioning using standardized instruments in children and adolescents with partial‐onset seizures treated with adjunctive levetiracetam in a randomized, placebo‐controlled trial. Epilepsy & Behavior 2010;18(3):291‐8. - PubMed
Peltola 2009 {published data only}
-
- Peltola J, Coetzee C, Jimenez F, Litovchenko T, Ramaratnam S, Zaslavaskiy L, et al. Once‐daily extended‐release levetiracetam as adjunctive treatment of partial‐onset seizures in patients with epilepsy: a double‐blind, randomized, placebo‐controlled trial. Epilepsia 2009;50(3):406‐14. - PubMed
Shorvon 2000 {published and unpublished data}
-
- Shorvon SD, Lowenthal A, Janz D, Bielen E, Loiseau P, for the European Levetiracetam Study Group. Multicenter double‐blind, randomized, placebo‐controlled trial of levetiracetam as add‐on therapy in patients with refractory partial seizures. Epilepsia 2000;41(9):1179‐86. - PubMed
-
- UCB. Evaluation of the efficacy and tolerability of UCB L059 (500 and 1000 mg b.i.d., tablets) add‐on treatment in refractory epileptic patients with partial onset seizures: a 32‐week double‐blind placebo‐controlled crossover multicenter trial. UCB S.A. Pharma Sector.
Tsai 2006 {published data only}
-
- Tsai JJ, Yen DJ, Hsih MS, Chen SS, Hiersemenzel R, Edrich P, et al. Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double‐blind, placebo‐controlled study. Epilepsia 2006;47(1):72‐81. - PubMed
Wu 2009 {published data only (unpublished sought but not used)}
-
- Wu XY, Hong Z, Wu X, Wu LW, Wang XF, Zhou D, et al. Multicenter double‐blind, randomized, placebo‐controlled trial of levetiracetam as add‐on therapy in Chinese patients with refractory partial‐onset seizures. Epilepsia 2009;50(3):398‐405. - PubMed
Xiao 2009 {published data only}
-
- Xiao Z, Li JM, Wang XF, Xiao F, Xi ZQ, Lv Y, et al. Efficacy and safety of levetiracetam (3,000 mg/day) as an adjunctive therapy in Chinese patients with refractory partial seizures. European Neurology 2009;61(4):233‐9. - PubMed
Zhou 2008 {published data only (unpublished sought but not used)}
-
- Zhou B, Zhang Q, Tian LY, Xiao J, Stefan H, Zhou D. Effects of levetiracetam as an add‐on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy & Behavior 2008;12(2):305‐10. - PubMed
References to studies awaiting assessment
Boon 2002 {published data only}
-
- Boon P, Chauvel P, Pohlmann‐Eden B, Otoul C, Wroe S. Dose‐response effect of levetiracetam 1000 and 2000mg/day in partial epilepsy. Epilepsy Research 2002;48(1):77‐89. [MEDLINE: ] - PubMed
N01221 {unpublished data only}
-
- UCB. A double‐blind, randomized, placebo‐controlled 5 parallel groups, confirmatory trial on the efficacy and safety of Levetiracetam used as add‐on therapy at doses of 0.5 to 3g/day in patients from 16 to 65 years with epilepsy with partial onset seizures under treatment with 1 to 3 anti‐epileptic drug(s). UCB Japan Co., Ltd. (RRCE07D0208). [CT Registry ID: NCT00280696]
Yagi 2010 {published data only}
-
- Yagi K, Kameyama S, Kaneko S, Murasaki M, Yamauchi T. [Multicenter, double‐blind, randomized, placebo‐controlled study of levetiracetam as add‐on therapy in Japanese patients with uncontrolled partial seizures]. Journal of the Japan Epilepsy Society. 2010;28(1):3‐16.
Zheng 2009 {published data only (unpublished sought but not used)}
-
- Zheng XZ, Wu SJ, Xia M, Li Q. Study on the therapeutic effect of levetiracetam as an additive therapy for refractory partial epilepsy and the relativity between levetiracetam and multidrug resistance gene: a randomised double‐blind placebo‐controlled trial. Chinese Journal of Contemporary Neurology and Neurosurgery 2009;9(2):173‐7.
Additional references
Asconapé 2001
-
- Asconapé JJ, Gerardot JM, Costa G. Behavioral changes associated with levetiracetam use in patients with epilepsy. Epilepsia 2001;42(Suppl 7):299.
Berg 2010
-
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005‐2009. Epilepsia 2010;51:676‐85. - PubMed
Brodie 2007
-
- Brodie MJ, Perucca E, Ryvlin P, Ben‐Menachem E, Meencke HJ, Levetiracetam Monotherapy Study Group. Comparison of levetiracetam and controlled‐release carbamazepine in newly diagnosed epilepsy. Neurology 2007;68(6):402‐8. - PubMed
Brodie 2010
-
- Brodie MJ. Antiepileptic drug therapy the story so far. Seizure 2010; Vol. 19, issue 10:650‐5. - PubMed
Burneo 2005
-
- Burneo JG, Tellez‐Zenteno J, Wiebe S. Understanding the burden of epilepsy in Latin America: a systematic review of its prevalence and incidence. Epilepsy Research 2005; Vol. 66, issue 1‐3:63‐74. - PubMed
Chaisewikul 2001
Cochrane 1998
-
- Cochrane HC, Marson AG, Baker GA, Chadwick DW. Neuropsychological outcomes in randomized controlled trials of antiepileptic drugs: a systematic review of methodology and reporting standards. Epilepsia 1998;39(10):1088‐97. - PubMed
Crepeau 2010
-
- Crepeau AZ, Treiman DM. Levetiracetam: a comprehensive review. Expert Review of Neurotherapeutics 2010;10(2):159‐71. - PubMed
Forsgren 2005
-
- Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe ‐ a systematic review. European Journal of Neurology 2005; Vol. 12, issue 4:245‐53. - PubMed
French 2004
-
- French JA, Kanner AM, Bautista J, Abou‐Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2004; Vol. 62, issue 8:1252‐60. - PubMed
French 2006
Gibbs 2006
-
- Gibbs JE, Walker MC, Cock HR. Levetiracetam: antiepileptic properties and protective effects on mitochondrial dysfunction in experimental status epilepticus. Epilepsia 2006;47:469–78. - PubMed
Gillard 2006
-
- Gillard M, Chatelain P, Fuks B. Binding characteristics of levetiracetam to synaptic vesicle protein 2A (SV2A) in human brain and in CHO cells expressing the human recombinant protein. European Journal of Pharmacology 2006; Vol. 536, issue 1‐2:102‐8. - PubMed
Guekht 2010
-
- Guekht AB, Korczyn AD, Bondareva IB, Gusev EI. Placebo responses in randomized trials of antiepileptic drugs. Epilepsy & Behavior 2010;17(1):64‐9. - PubMed
Hauser 1993
-
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935‐1984. Epilepsia 1993;34(3):453‐68. - PubMed
Higgins 2005
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd.
Kerr 2011
-
- Kerr C, Nixon A, Angalakuditi M. The impact of epilepsy on children and adult patients' lives: development of a conceptual model from qualitative literature. Seizure 2011;20(10):764‐74. - PubMed
Krauss 2011
Kwan 2010
-
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51(6):1069‐77. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lukyanetz 2002
-
- Lukyanetz EA, Shkryl VM, Kostyuk PG. Selective blockade of N‐type calcium channels by levetiracetam. Epilepsia 2002; Vol. 43, issue 1:9‐18. - PubMed
Lynch 2004
-
- Lynch BA, Lambeng N, Nocka K, Kensel‐Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proceedings of the National Academy of Sciences of the United States of America 2004; Vol. 101, issue 26:9861‐6. - PMC - PubMed
Mac 2007
-
- Mac TL, Tran DS, Quet F, Odermatt P, Preux PM, Tan CT. Epidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic review. Lancet Neurology 2007; Vol. 6, issue 6:533‐43. - PubMed
Maguire 2011
McCullagh 1989
-
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd Edition. London: Chapman and Hall, 1989.
NICE 2012
-
- National Institute for Clinical Excellence. NICE Clinical Guideline 137. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care, January 2012. www.guidance.nice.org.uk/CG137 (accessed 3 August 2012).
Niespodziany 2001
-
- Niespodziany I, Klitgaard H, Margineanu DG. Levetiracetam inhibits the high‐voltage‐activated Ca(2+) current in pyramidal neurones of rat hippocampal slices. Neuroscience Letters 2001; Vol. 306, issue 1‐2:5‐8. - PubMed
Pellock 2001
-
- Pellock JM, Glauser TA, Bebin EM, Fountain NB, Ritter FJ, Coupez RM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia 2001; Vol. 42, issue 12:1574‐9. - PubMed
Penovich 2004
Preux 2005
-
- Preux PM, Druet‐Cabanac M. Epidemiology and aetiology of epilepsy in sub‐Saharan Africa. Lancet Neurology 2005; Vol. 4, issue 1:21‐31. - PubMed
Rheims 2011
-
- Rheims S, Perucca E, Cucherat M, Ryvlin P. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta‐analysis. Epilepsia 2011;52(2):219‐33. - PubMed
Rigo 2002
SANAD‐II
-
- SANAD‐II. A pragmatic randomised controlled trial comparing the effectiveness and cost effectiveness of levetiracetam and zonisamide versus standard treatments for epilepsy: a comparison of Standard And New Antiepileptic Drugs (SANAD‐II). www.hta.ac.uk/2562 (accessed 3 August 2012). - PMC - PubMed
Sander 1996
Schünemann 2009
-
- Schünemann H, Brożek J, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group, 2009. Available from www.cc‐ims.net/gradepro (accessed 3 August 2012).
Shorvon 1996
-
- Shorvon SD. The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia 1996;37(Suppl 2):S1‐3. - PubMed
Trinka 2011
-
- Trinka E. What is the evidence to use new intravenous AEDs in status epilepticus?. Epilepsia 2011;52(Suppl 8):35‐8. - PubMed
Verrotti 2010
-
- Verrotti A, D'Adamo E, Parisi P, Chiarelli F, Curatolo P. Levetiracetam in childhood epilepsy. Paediatric Drugs 2010;12(3):177‐86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

