Robot assistant versus human or another robot assistant in patients undergoing laparoscopic cholecystectomy
- PMID: 22972093
- PMCID: PMC4212273
- DOI: 10.1002/14651858.CD006578.pub3
Robot assistant versus human or another robot assistant in patients undergoing laparoscopic cholecystectomy
Abstract
Background: The role of a robotic assistant in laparoscopic cholecystectomy is controversial. While some trials have shown distinct advantages of a robotic assistant over a human assistant others have not, and it is unclear which robotic assistant is best.
Objectives: The aims of this review are to assess the benefits and harms of a robot assistant versus human assistant or versus another robot assistant in laparoscopic cholecystectomy, and to assess whether the robot can substitute the human assistant.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (until February 2012) for identifying the randomised clinical trials.
Selection criteria: Only randomised clinical trials (irrespective of language, blinding, or publication status) comparing robot assistants versus human assistants in laparoscopic cholecystectomy were considered for the review. Randomised clinical trials comparing different types of robot assistants were also considered for the review.
Data collection and analysis: Two authors independently identified the trials for inclusion and independently extracted the data. We calculated the risk ratio (RR) or mean difference (MD) with 95% confidence interval (CI) using the fixed-effect and the random-effects models based on intention-to-treat analysis, when possible, using Review Manager 5.
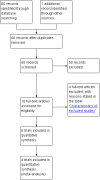
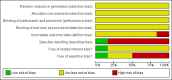
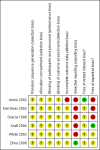
Main results: We included six trials with 560 patients. One trial involving 129 patients did not state the number of patients randomised to the two groups. In the remaining five trials 431 patients were randomised, 212 to the robot assistant group and 219 to the human assistant group. All the trials were at high risk of bias. Mortality and morbidity were reported in only one trial with 40 patients. There was no mortality or morbidity in either group. Mortality and morbidity were not reported in the remaining trials. Quality of life or the proportion of patients who were discharged as day-patient laparoscopic cholecystectomy patients were not reported in any trial. There was no significant difference in the proportion of patients who required conversion to open cholecystectomy (2 trials; 4/63 (weighted proportion 6.4%) in the robot assistant group versus 5/70 (7.1%) in the human assistant group; RR 0.90; 95% CI 0.25 to 3.20). There was no significant difference in the operating time between the two groups (4 trials; 324 patients; MD 5.00 minutes; 95% CI -0.55 to 10.54). In one trial, about one sixth of the laparoscopic cholecystectomies in which a robot assistant was used required temporary use of a human assistant. In another trial, there was no requirement for human assistants. One trial did not report this information. It appears that there was little or no requirement for human assistants in the other three trials. There were no randomised trials comparing one type of robot versus another type of robot.
Authors' conclusions: Robot assisted laparoscopic cholecystectomy does not seem to offer any significant advantages over human assisted laparoscopic cholecystectomy. However, all trials had a high risk of systematic errors or bias (that is, risk of overestimation of benefit and underestimation of harm). All trials were small, with few or no outcomes. Hence, the risk of random errors (that is, play of chance) is high. Further randomised trials with low risk of bias or random errors are needed.
Conflict of interest statement
None known
Figures


Update of
-
Robot assistant for laparoscopic cholecystectomy.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD006578. doi: 10.1002/14651858.CD006578.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Sep 12;(9):CD006578. doi: 10.1002/14651858.CD006578.pub3. PMID: 19160289 Updated.
References
References to studies included in this review
Aiono 2002 {published data only}
-
- Aiono S, Gilbert JM, Soin B, Finlay PA, Gordan A. Controlled trial of the introduction of a robotic camera assistant (EndoAssist) for laparoscopic cholecystectomy. Surgical Endoscopy 2002;16(9):1267‐70. - PubMed
Den Boer 2002 {published data only}
-
- Boer KT, Bruijn M, Jaspers JE, Stassen LPS, Erp WFM, Jansen A, et al. Time‐action analysis of instrument positioners in laparoscopic cholecystectomy: a multicenter prospective randomized trial. Surgical Endoscopy 2002;16(1):142‐7. - PubMed
Gracia 1996 {published data only}
-
- Gracia CR, Estakhri ME, Ramon S. Evaluation of robotic laparoscope positioner. Proceedings of the Eighth Annual International Conference for Minimally Invasive Therapy, Milan, Italy, September. 1996:O118.
Kraft 2004 {published data only}
-
- Kraft BM, Jager C, Kraft K, Leibl BJ, Bittner R. The AESOP robot system in laparoscopic surgery: increased risk or advantage for surgeon and patient?. Surgical Endoscopy 2004;18(8):1216‐23. - PubMed
White 2003 {published data only}
-
- White AP, Carhajal‐Ramus A, Gracia C, Nunez‐Gonzalez E, Bailey R, Broderick T, et al. A prospective randomized study of the ZEUS robotic surgical system for laparoscopic cholecystectomy. Surgical Endoscopy 2003;17(Suppl 1):S207.
Zhou 2006 {published data only}
-
- Zhou HX, Guo YH, Yu XF, Bao SY, Liu JL, Zhang Y, et al. Zeus robot‐assisted laparoscopic cholecystectomy in comparison with conventional laparoscopic cholecystectomy. Hepatobiliary & Pancreatic Diseases International 2006;5(1):115‐8. - PubMed
References to studies excluded from this review
Heemskerk 2005 {published data only}
-
- Heemskerk J, Dam R, Gemert WG, Beets GL, Greve JW, Jacobs MJ, et al. First results after introduction of the four‐armed da Vinci Surgical System in fully robotic laparoscopic cholecystectomy. Digestive Surgery 2005;22(6):426‐31. - PubMed
Hourmont 2003 {published data only}
-
- Hourmont K, Chung W, Pereira S, Wasielewski A, Davies R, Ballantyne GH. Robotic versus telerobotic laparoscopic cholecystectomy: duration of surgery and outcomes. The Surgical Clinics of North America 2003;83(6):1445‐62. - PubMed
Kornprat 2006 {published data only}
-
- Kornprat P, Werkgartner G, Cerwenka H, Bacher H, El‐Shabrawi A, Rehak P, et al. Prospective study comparing standard and robotically assisted laparoscopic cholecystectomy. Langenbecks Archives of Surgery 2006;391(3):216‐21. - PubMed
Nio 2004 {published data only}
-
- Nio D, Bemelman WA, Busch OR, Vrouenraets BC, Gouma DJ. Robot‐assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy: a comparative study. Surgical Endoscopy 2004;18(3):379‐82. - PubMed
Tanoue 2006 {published data only}
-
- Tanoue K, Yasunaga T, Kobayashi E, Miyamoto S, Sakuma I, Dohi T, et al. Laparoscopic cholecystectomy using a newly developed laparoscope manipulator for 10 patients with cholelithiasis. Surgical Endoscopy 2006; Vol. 20, issue 5:753‐6. - PubMed
Additional references
Bakken 2004
-
- Bakken IJ, Skjeldestad FE, Mjåland O, Johnson E. Cholecystectomy in Norway 1990‐2002 [Kolecystektomi i Norge i 1990‐2002]. Tidsskrift for den Norske Laegeforening 2004;124(18):2376‐8. - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Egger 1997
Fullarton 1994
Gluud 2012
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2012, Issue 5. Art. No.: LIVER.
Gurusamy 2009a
-
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. The British Journal of Surgery 2009b;96(4):342‐9. - PubMed
Halldestam 2004
-
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. The British Journal of Surgery 2004;91(6):734‐8. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1996
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Jørgensen 1987
-
- Jørgensen T. Prevalence of gallstones in a Danish population. American Journal of Epidemiology 1987;126(5):912‐21. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Livingston 2004
-
- Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. American Journal of Surgery 2004;188(3):205‐11. - PubMed
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Mjäland 1998
-
- Mjäland O, Adamsen S, Hjelmquist B, Ovaska J, Buanes T. Cholecystectomy rates, gallstone prevalence, and handling of bile duct injuries in Scandinavia. A comparative audit. Surgical Endoscopy 1998;12(12):1386‐9. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Muhrbeck 1995
-
- Muhrbeck O, Ahlberg J. Prevalence of gallstone disease in a Swedish population. Scandinavian Journal of Gastroenterology 1995;30(11):1125‐8. - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
NIH 1992
-
- NIH consensus statement on gallstones and laparoscopic cholecystectomy. http://consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm 1992 (accessed 2 July 2012). - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Rutledge 2000
-
- Rutledge D, Jones D, Rege R. Consequences of delay in surgical treatment of biliary disease. American Journal of Surgery 2000;180(6):466‐9. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Wood 2008
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

