Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections
- PMID: 22972110
- PMCID: PMC6464976
- DOI: 10.1002/14651858.CD007498.pub2
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections
Update in
-
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections.Cochrane Database Syst Rev. 2017 Oct 12;10(10):CD007498. doi: 10.1002/14651858.CD007498.pub3. Cochrane Database Syst Rev. 2017. PMID: 29025194 Free PMC article.
Abstract
Background: Acute respiratory infections (ARIs) comprise a large and heterogeneous group of infections including bacterial, viral and other aetiologies. In recent years, procalcitonin - the prohormone of calcitonin - has emerged as a promising marker for the diagnosis of bacterial infections and for improving decisions about antibiotic therapy. Several randomised controlled trials (RCTs) have demonstrated the feasibility of using procalcitonin for starting and stopping antibiotics in different patient populations with acute respiratory infections and different settings ranging from primary care to emergency departments (EDs), hospital wards and intensive care units (ICUs).
Objectives: The aim of this systematic review based on individual patient data was to assess the safety and efficacy of using procalcitonin for starting or stopping antibiotics over a large range of patients with varying severity of ARIs and from different clinical settings.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2011, Issue 2) which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to May 2011) and EMBASE (1974 to May 2011) to identify suitable trials.
Selection criteria: We included RCTs of adult participants with ARIs who received an antibiotic treatment either based on a procalcitonin algorithm or usual care/guidelines. Trials were excluded if they exclusively focused on paediatric patients or if they used procalcitonin for another purpose than to guide initiation and duration of antibiotic treatment.
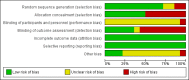
Data collection and analysis: Two teams of review authors independently evaluated the methodology and extracted data from primary studies. The primary endpoints were all-cause mortality and treatment failure at 30 days. For the primary care setting, treatment failure was defined as death, hospitalisation, ARI-specific complications, recurrent or worsening infection, and patients reporting any symptoms of an ongoing respiratory infection at follow-up. For the ED setting, treatment failure was defined as death, ICU admission, re-hospitalisation after index hospital discharge, ARI-associated complications, and recurrent or worsening infection within 30 days of follow-up. For the ICU setting, treatment failure was defined as death within 30 days of follow-up. Secondary endpoints were antibiotic use (initiation of antibiotics, duration of antibiotics and total exposure to antibiotics (total amount of antibiotic days divided by total number of patients)), length of hospital stay for hospitalised patients, length of ICU stay for critically ill patients, and number of days with restricted activities within 14 days after randomisation for primary care patients.For the two co-primary endpoints of all-cause mortality and treatment failure, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) using multivariable hierarchical logistic regression. The hierarchical regression model was adjusted for age and clinical diagnosis as fixed-effect. The different trials were added as random-effects into the model. We fitted corresponding linear regression models for antibiotic use. We conducted sensitivity analyses stratified by clinical setting and ARI diagnosis to assess the consistency of our results.
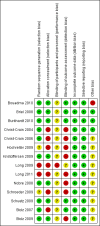
Main results: We included 14 trials with 4221 participants. There were 118 deaths in 2085 patients (5.7%) assigned to procalcitonin groups compared to 134 deaths in 2126 control patients (6.3%) (adjusted OR 0.94, 95% CI 0.71 to 1.23). Treatment failure occurred in 398 procalcitonin group patients (19.1%) and in 466 control patients (21.9%). Procalcitonin guidance was not associated with increased mortality or treatment failure in any clinical setting, or ARI diagnosis. These results proved robust in various sensitivity analyses. Total antibiotic exposure was significantly reduced overall (median (interquartile range) from 8 (5 to 12) to 4 (0 to 8) days; adjusted difference in days, -3.47, 95% CI -3.78 to -3.17, and across all the different clinical settings and diagnoses.
Authors' conclusions: Use of procalcitonin to guide initiation and duration of antibiotic treatment in patients with ARI was not associated with higher mortality rates or treatment failure. Antibiotic consumption was significantly reduced across different clinical settings and ARI diagnoses. Further high-quality research is needed to confirm the safety of this approach for non-European countries and patients in intensive care. Moreover, future studies should also establish cost-effectiveness by considering country-specific costs of procalcitonin measurement and potential savings in consumption of antibiotics and other healthcare resources, as well as secondary cost savings due to lower risk of side effects and reduced antimicrobial resistance.
Conflict of interest statement
Funding: this study was partly funded by unrestricted research grants from BRAHMS/Thermo fisher Scientific, the Gottfried and Julia Bangerter‐Rhyner‐Foundation, the Swiss Foundation for Grants in Biology and Medicine (SSMBS, PASMP3‐127684/1) and Santésuisse to cover salary time related to this study. The sponsors had no role in the study design, data collection, data analysis or data interpretation, or writing of the report.
No commercial sponsor had any involvement in design and conduct of this study, namely collection, management, analysis and interpretation of the data; and preparation, decision to submit, review or approval of the manuscript.
Drs. Schuetz, Christ‐Crain and Mueller received support from BRAHMS and bioMérieux to attend meetings and fulfilled speaking engagements. Dr. Mueller has served as a consultant and received research support. Drs. Stolz, Burkhardt and Tamm received research support from BRAHMS. Drs. Welte and Schroeder received lecture fees and research support from BRAHMS. Dr Luyt received lecture fees from Brahms and Merck Sharp & Dohme‐Chibret. Dr Chastre received consulting and lecture fees from Pfizer, Brahms, Wyeth, Johnson & Johnson, Nektar‐Bayer and Arpida. Dr Wolff received consulting and lectures fees from Merck Sharp & Dohme‐Chibret, Janssen–Cilag, Gilead and Astellas. Dr. Tubach received research grants from Abbott, Astra‐Zeneca, Pfizer and Schering Plough. All other authors declare that the answer to the questions on the competing interest form are all 'No' and therefore have nothing to declare.
Figures






Comment in
-
ACP Journal Club. Meta-analysis: procalcitonin-guided antibiotic therapy reduces treatment failure in acute respiratory infection.Ann Intern Med. 2013 Feb 19;158(4):JC5. doi: 10.7326/0003-4819-158-4-201302190-02005. Ann Intern Med. 2013. PMID: 23420253 No abstract available.
References
References to studies included in this review
-
- Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463‐74. - PubMed
-
- Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Archives of Internal Medicine 2008;168(18):2000‐7. - PubMed
-
- Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wegscheider K, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. European Respiratory Journal 2010;36(3):601‐7. - PubMed
-
- Christ‐Crain M, Jaccard‐Stolz D, Bingisser R, Gencay M, Huber P, Tamm M, et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet 2004;1363(9409):600‐7. - PubMed
-
- Christ‐Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2006;174(1):84‐93. - PubMed
References to studies excluded from this review
-
- Esposito S, Tagliabue C, Picciolli I, Semino M, Sabatini C, Consolo S, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respiratory Medicine 2011;105(12):1939‐45. - PubMed
-
- Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Critical Care Medicine 2011;39(7):1792‐9. - PubMed
-
- Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Critical Care Medicine 2011;39(9):2048‐58. - PubMed
-
- Jones AE, Fiechtl JF, Brown MD, Ballew JJ, Kline JA. Procalcitonin test in the diagnosis of bacteremia: a meta‐analysis. Annals of Emergency Medicine 2007;50(1):34‐41. - PubMed
-
- Kook JL, Chao SR, Le J, Robinson PA. Impact of the use of procalcitonin assay in hospitalised patients with pneumonia at a community care hospital. Infection Control and Hospital Epidemiology 2012;33(4):424‐6. - PubMed
References to studies awaiting assessment
-
- Benni I. Procalcitonin‐aided antibiotic therapy management in hospital‐acquired pneumonia and healthcare‐associated pneumonia: A randomised controlled trial (the prohap study). Poster at the Respirology. Conference: 16th Congress of the Asian Pacific Society of Respirology Shanghai, China. Shanghai, China, 2011; Vol. 16:68.
References to ongoing studies
-
- Procalcitonin level to discontinue antibiotics on ICU patients with no obvious site of infection. Ongoing study 29 November 2006.
-
- Impact of procalcitonin on the management of children aged 1 to 36 month presenting with a fever without a source. Ongoing study November 2006. - PubMed
-
- Placebo controlled trial of sodium selenite and procalcitonin guided antimicrobial therapy in severe sepsis (SISPCT). Ongoing study 28 January 2009.
-
- Neonatal Procalcitonin Intervention Study (NeoPInS). Ongoing study 2 March 2009.
-
- Use of procalcitonin level for guidance of the treatment of suspected community acquired pneumonia. Ongoing study June 2009.
Additional references
-
- Classen DC, Jaser L, Budnitz DS. Adverse drug events among hospitalized Medicare patients: epidemiology and national estimates from a new approach to surveillance. Joint Commission Journal on Quality and Patient Safety/Joint Commission Resources 2010;36(1):12‐21. - PubMed
-
- Dixon RE. Economic costs of respiratory tract infections in the United States. American Journal of Medicine 1985;78(6B):45‐51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

