Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease
- PMID: 22972134
- PMCID: PMC8935978
- DOI: 10.1002/14651858.CD009157.pub2
Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease
Abstract
Background: Tiotropium and long-acting beta(2)-agonists (LABAs) are both accepted in the routine management for people with stable chronic obstructive pulmonary disease (COPD). There are new studies which have compared tiotropium with LABAs, including some that have evaluated recently introduced LABAs.
Objectives: To compare the relative clinical effects of tiotropium bromide alone versus LABA alone, upon measures of quality of life, exacerbations, lung function and serious adverse events, in people with stable COPD.To critically appraise and summarise current evidence on the costs and cost-effectiveness associated with tiotropium compared to LABA in people with COPD.
Search methods: We identified randomised controlled trials (RCTs) from the Cochrane Airways Group Specialised Register of trials and economic evaluations from searching NHS EED and HEED (date of last search February 2012). We found additional trials from web-based clinical trial registers.
Selection criteria: We included RCTs and full economic evaluations if they compared effects of tiotropium alone with LABAs alone in people with COPD. We allowed co-administration of standard COPD therapy.
Data collection and analysis: Two review authors independently assessed studies for inclusion, then extracted data on study quality and outcomes. We contacted study authors and trial sponsors for additional information. We analysed data using the Cochrane Review Manager(RevMan 5.1) software.
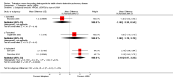
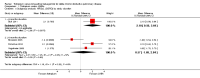
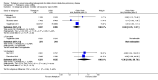
Main results: Seven clinical studies totalling 12,223 participants with COPD were included in the review. The studies used similar designs and were generally of good methodological quality. Inclusion criteria for RCTs were similar across the included studies, although studies varied in terms of smoking history and COPD severity of participants. They compared tiotropium (which was delivered by HandiHaler in all studies) with salmeterol (four studies, 8936 participants), formoterol (one study, 431 participants) and indacaterol (two studies, 2856 participants). All participants were instructed to discontinue anticholinergic or long-acting beta(2)-agonist bronchodilators during treatment, but could receive inhaled corticosteroids (ICS) at a stable dose. Study duration ranged from 3 to 12 months. We extracted data for 11,223 participants. In general, the treatment groups were well matched at baseline. Overall, the risk of bias across the included RCTs was low.In the analysis of the primary outcomes in this review, a high level of heterogeneity amongst studies meant that we did not pool data for St George's Respiratory Questionnaire quality of life score. Subgroup analyses based on the type of LABA found statistically significant differences among effects on quality of life depending on whether tiotropium was compared with salmeterol, formoterol or indacaterol. Tiotropium reduced the number of participants experiencing one or more exacerbations compared with LABA (odds ratio (OR) 0.86; 95% confidence interval (CI) 0.79 to 0.93). For this outcome, there was no difference seen among the different types of LABA. There was no statistical difference in mortality observed between the treatment groups.For secondary outcomes, tiotropium was associated with a reduction in the number of COPD exacerbations leading to hospitalisation compared with LABA treatment (OR 0.87; 95% 0.77 to 0.99), but not in the overall rate of all-cause hospitalisations. There was no statistically significant difference in forced expiratory volume in one second (FEV(1)) or symptom score between tiotropium and LABA-treated participants. There was a lower rate of non-fatal serious adverse events recorded with tiotropium compared with LABA (OR 0.88; 95% CI 0.78 to 0.99). The tiotropium group was also associated with a lower rate of study withdrawals (OR 0.89; 95% CI 0.81 to 0.99).We identified six full economic evaluations assessing the cost and cost-effectiveness of tiotropium and salmeterol. The studies were based on an economic model or empirical analysis of clinical data from RCTs. They all looked at maintenance costs and the costs for COPD exacerbations, including respiratory medications and hospitalisations. The setting for the evaluations was primary and secondary care in the UK, Greece, Netherlands, Spain and USA. All the studies estimated tiotropium to be superior to salmeterol based on better clinical outcomes (exacerbations or quality of life) and/or lower total costs. However, the authors of all evaluations reported there was substantial uncertainty around the results.
Authors' conclusions: In people with COPD, the evidence is equivocal as to whether or not tiotropium offers greater benefit than LABAs in improving quality of life; however, this is complicated by differences in effect among the LABA types. Tiotropium was more effective than LABAs as a group in preventing COPD exacerbations and disease-related hospitalisations, although there were no statistical differences between groups in overall hospitalisation rates or mortality during the study periods. There were fewer serious adverse events and study withdrawals recorded with tiotropium compared with LABAs. Symptom improvement and changes in lung function were similar between the treatment groups. Given the small number of studies to date, with high levels of heterogeneity among them, one approach may be to give a COPD patient a substantial trial of tiotropium, followed by a LABA (or vice versa), then to continue prescribing the long-acting bronchodilator that the patient prefers. Further studies are needed to compare tiotropium with different LABAs, which are currently ongoing. The available economic evidence indicates that tiotropium may be cost-effective compared with salmeterol in several specific settings, but there is considerable uncertainty around this finding.
Conflict of interest statement
None known.
Figures
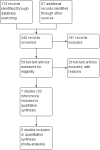







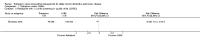












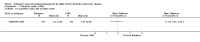


Update of
References
References to studies included in this review
Briggs 2005 {published data only}
-
- Briggs Jr D, Covelli H, Lapidus R, Bhattacharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared to salmeterol in COPD patients [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:C22 Poster 511.
-
- Briggs Jr DD, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulmonary Pharmacology & Therapeutics 2005;18(6):397‐404. - PubMed
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. Proceedings of the American Thoracic Society. 2006:A110 [Poster J10].
-
- Langley J, Briggs Jr D, Bhattacharya S, Kesten S, Cassino C. Spirometric outcomes of COPD patients with tiotropium compared with salmeterol according to previous maintenance long acting beta‐agonist use [Abstract]. European Respiratory Journal 2004;24(Suppl 48):289s.
Brusasco 2003 {published data only}
-
- Bateman ED, Hodder R, Miravitlles M, Lee A, Towse L, Serby C. A comparative trial of tiotropium, salmeterol and placebo: health‐related quality of life. European Respiratory Journal 2001;18(Suppl 33):26s.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders following treatment with tiotropium and salmeterol in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 Poster 420.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. Proceedings of the American Thoracic Society. 2006:A110 [Poster J10].
-
- Brusasco V, Thompson P, Vincken W, Lee A, Towse L, Witek TJ. Improvement of dyspnea following of six months treatment with tiotropium but not with salmeterol in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):26s.
Burl 2011 {published and unpublished data}
-
- Buhl R, Dunn LJ, Disdier C, Lassen C, Amos C, Henley M, et al. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD. European Respiratory Journal 2011;38:797‐803. - PubMed
-
- Dunn LJ, Buhl R, Lassen C, Henley M, Kramer B. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD [Abstract]. Chest. 2010; Vol. 138:719A. - PubMed
Donohue 2010 {published data only}
-
- Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, et al. Once‐daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. American Journal of Respiratory & Critical Care Medicine 2010;182(2):155‐62. - PubMed
-
- Fogarty C, Hebert J, Iqbal A, Owen R, Higgins M, Kramer B. Indacaterol once‐daily provides effective 24‐h bronchodilation in COPD patients: a 26‐week evaluation vs placebo and tiotropium [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[P2025].
-
- Jones PW, Mahler DA, Gale R, Owen R, Kramer B. Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respiratory Medicine 2011;105(6):892‐9. - PubMed
-
- Lotvall J, Cosio BG, Iqbal A, Swales J, Owen R, Kramer B, et al. Indacaterol once‐daily improves day and night‐time symptom control in COPD patients: a 26‐week study versus placebo and tiotropium [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[P2029].
-
- Mahler D, Palange P, Iqbal A, Owen R, Higgins M, Kramer B. Indacaterol once‐daily improves dyspnoea in COPD patients: a 26‐week placebo‐controlled study with open‐label tiotropium comparison [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[E4360].
Gani 2010 {published data only}
Mahmud 2007 {published data only}
-
- Mahmud AM, Gupta DK, Khan AS, Hassan R, Hossain A, Rahman M, et al. Comparison of once daily tiotropium with twice daily salmeterol in Bangladeshi patients with moderate COPD [Abstract]. Respirology 2007;12(Suppl 4):A211.
Maniadakis 2006 {published data only}
-
- Maniadakis N, Tzanakis N, Fragoulakis V, Hatzikou M, Siafakas N. Economic evaluation of tiotropium and salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) in Greece. Current Medical Research and Opinion 2006;22(8):1599‐607. - PubMed
Naik 2010 {published data only}
Oba 2007 {published data only}
-
- Oba Y. Cost‐effectiveness of long‐acting bronchodilators for chronic obstructive pulmonary disease. Mayo Clinic Proceedings 2007;82(5):575‐82. - PubMed
Oostenbrink 2005 {published data only}
-
- Oostenbrink JB, Rutten‐van Molken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost‐effectiveness of bronchodilator therapy in COPD patients in different countries. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research 2005;8(1):32‐46. - PubMed
Rutten‐van Molken 2007 {published data only}
-
- Rutten‐van Molken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5‐year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. The European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 2007;8(2):123‐35. - PMC - PubMed
Vogelmeier 2008 {published data only}
-
- Arievich H, Potena A, Fonay K, Vogelmeier CF, Overend T, Smith J, et al. Formoterol given either alone or together with tiotropium reduces the rate of exacerbations in stable COPD patients [Abstract]. European Respiratory Journal 2006;28(Suppl 50):440s [P2514].
-
- Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono‐ and combination therapy with tiotropium in patients with COPD: a 6‐month study. Respiratory Medicine 2008;102(11):1511‐20. - PubMed
-
- Vogelmeier CF, Harari SA, Fonay K, Beier J, Overend T, Till D, et al. Formoterol and tiotropium both improve lung function in stable COPD patients with some additional benefit when given together [Abstract]. European Respiratory Journal 2006;28(Suppl 50):429s [P2506].
Vogelmeier 2011 {published data only}
-
- Beeh KM, Vogelmeier C, Rutten‐van Mölken M, Rabe KF, Glaab T, et al. Tiotropium vs salmeterol in GOLD II and maintenance‐naive COPD patients: subgroup analyses of POET‐COPD™ trial [Abstract]. European Respiratory Society Annual Congress, Amsterdam, The Netherlands, September 24‐28 2011; Vol. 38, issue 55:20s [P251].
-
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten‐van Mölken MPMH, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. New England Journal of Medicine 2011;364(12):1093‐103. - PubMed
-
- Vogelmeier C, Rabe K, Beeh KM, Glaab T, Schmidt H, Rutten‐van Mölken M, et al. Tiotropium reduces exacerbations versus salmeterol irrespective of baseline ICS treatment in the Poet‐COPD; Study [Abstract]. American Journal of Respiratory and Critical Care Medicine. 2011; Vol. 183:A1600.
-
- Vogelmeier CF, Beeh KM, Rutten‐van Molken M, Glaab T, Schmidt H, Hederer B. Baseline characteristics according to gender in a large exacerbations trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4495.
References to studies excluded from this review
Barnes 2010 {published data only}
-
- Barnes P, Pocock S, Magnussen H, Iqbal A, Lawrence D, Kramer B, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. American Journal of Respiratory and Critical Care Medicine. 2009; Vol. 179, issue Meeting Abstracts:A4543. - PubMed
-
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulmonary Pharmacology and Therapeutics 2010;23(3):165‐71. - PubMed
-
- Fogarty C, Worth H, Hebert J, Iqbal A, Owen R, Higgins M, et al. Sustained 24‐h bronchodilation with indacaterol once‐daily in COPD: a 26‐week efficacy and safety study. American Journal of Respiratory and Critical Care Medicine. 2009; Vol. 179, issue 1 Meeting Abstracts:A4547.
-
- Rennard S, Bantje T, Centanni S, Chanez P, Chuchalin A, D'Urzo A, et al. A dose‐ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respiratory Medicine 2008;102(7):1033‐44. - PubMed
-
- Rennard SI, Chapman KR, Luthra A, Swales J, Lassen C, Owen R, et al. Once‐daily indacaterol provides effective bronchodilation over 1 year of treatment in patients with chronic obstructive pulmonary disease (COPD). Chest 2009;136(4):4S‐f.
Chapman 2010 {published data only}
-
- Chapman KR, Fogarty CM, Peckitt C, Lassen C, Kramer B. Patient handling and preference for the single‐dose dry‐powder inhalers used with indacaterol and tiotropium [Abstract]. Chest. 2010; Vol. 138:470A.
Di Marco 2006 {published data only}
-
- Marco F, Carlucci P, Ccarlucci P, Verga M, Mondoni M, Pistone A, et al. A single dose of formoterol (FOR) and tiotropium (TIO) reduce hyperinflation in COPD patients [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: [P1844].
-
- Marco F, Verga M, Santus P, Cazzola M, Mondoni M, Belloi E, et al. Formoterol (Modulite ®), tiotropium and their combination in patients with acute exacerbations of chronic bronchitis: preliminary data [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1905.
-
- Marco F, Verga M, Santus P, Morelli N, Cazzola M, Centanni S. Effect of formoterol, tiotropium, and their combination in patients with acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Respiratory Medicine 2006;100(11):1925‐32. - PubMed
Donohue 2011 {published data only}
-
- Donohue J, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. Dose‐related efficacy of GSK573719: a long‐acting muscarinic receptor antagonist (LAMA) with sustained 24‐hour activity in COPD [Abstract]. Chest 2011; Vol. 140, issue 4:1043A.
Golubev 2006 {published data only}
-
- Golubev L, Gorbunova M, Babak S, Tatarskiy. Sleep architecture in COPD patients during salmeterol versus tiotropium bromide treatment [Abstract]. European Respiratory Journal 2006;28(Suppl 50):199s [P1197].
Gross 2003 {published data only}
-
- Gross NJ, Paulson D, Kennedy D, Korducki L, Kesten S. A comparison of maintenance tiotropium and salmeterol on arterial blood gas tensions in patients with COPD [Abstract]. Respiratory Care 2003;48(11):1081.
Meyer 2008 {published data only}
-
- Meyer T, Brand P, Reitmeir P, Scheuch G. Effect of formoterol, salbutamol and tiotropium bromide on the efficacy of lung clearance in patients with COPD [Abstract]. American Thoracic Society International Conference, May 16‐21, 2008, Toronto. 2008:A646[#F1].
Meyer 2011 {published data only}
-
- Meyer T, Reitmeir P, Brand P, Herpich C, Sommerer K, Schulze A, et al. Effects of formoterol and tiotropium bromide on mucus clearance in patients with COPD. Respiratory Medicine 2011; Vol. 105, issue 6:900‐6. - PubMed
Reisner 2011 {published data only}
-
- Reisner C, Fogarty C, Spangenthal S, Dunn L, Kerwin EM, Quinn D, et al. Novel combination of glycopyrrolate and formoterol MDI (GFF‐MDI) provides superior bronchodilation compared to its components administered alone, tiotropium DPI, and formoterol DPI in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011; Vol. 183, issue Meeting Abstracts:A6435.
-
- Reisner C, St. Rose E, Strom S, Fischer T, Golden M, Thomas M, et al. Fixed combination of glycopyrrolate and formoterol MDI (GFF‐MDI) demonstrates superior inspiratory capacity (IC) compared to tiotropium DPI (Tio) following 7 days dosing, in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. European Respiratory Society Annual Congress, Amsterdam, The Netherlands, September 24‐28 2011; Vol. 38, issue 55:150s [P879].
Rossi 2012 {published data only}
-
- Rossi A, Centanni S, Cerveri I, Gulotta C, Foresi A, Cazzola M, et al. Acute effects of indacaterol on lung hyperinflation in moderate COPD: a comparison with tiotropium. Respiratory Medicine 2012; Vol. 106, issue 1:84‐90. - PubMed
Tashkin 2009 {published data only}
-
- Tashkin D, Donohue J, Mahler D, Huang H, Schaefer K, Hanrahan J, et al. Effect of arformoterol twice daily, tiotropium once daily, and their combination in subjects with COPD [Abstract]. Chest 2008;134(4):104003s. - PubMed
-
- Tashkin DP, Donohue JF, Mahler DA, Huang H, Goodwin E, Schaefer K, et al. Effects of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPD. Respiratory Medicine 2009;103(4):516‐24. - PubMed
ten Hacken 2007 {published data only}
-
- Hacken NH, Haart H, Grevink R, Thompson S, Roemer W, Postma DS. Bronchodilation improves endurance but not muscular efficiency in COPD [Abstract]. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California, USA. 2007:Poster #M72.
van der Vaart 2011 {published data only}
van Noord 2003 {published data only}
-
- Noord JA, Aumann J, Janssens E, Folgering H, Mueller A, Cornelissen JPG. Tiotropium maintenance therapy in patients with COPD and the 24‐h spirometric benefit of adding once or twice daily formoterol during 2‐week treatment periods [abstract]. American Thoracic Society 99th International Conference. 2003:A035 Poster D82.
van Noord 2005 {published data only}
-
- Noord JA, Aumann J, Janssens E, Mueller A, Cornelissen PJ. Comparison of once daily tiotropium, twice daily formoterol and the free combination, once daily, in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 Poster 421.
-
- Noord JA, Aumann J, Janssens E, Mueller A, Cornelissen PJG. A comparison of the 24 hour bronchodilator effect of tiotropium QD [T10] salmeterol BID [SALM] or their combination in COPD [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:[B93] [Poster: 311].
-
- Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. European Respiratory Journal 2005;26(2):214‐22. - PubMed
van Noord 2006 {published data only}
-
- Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 2006;129(3):509‐17. - PubMed
-
- Noord JA, Smeets JJ, Otte A, Bindels H, Mueller A, Cornellissen PJG. The effect of tiotropium, salmeterol and it's combination on dynamic hyperinflation in COPD [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:[B93] [Poster: 310].
References to studies awaiting assessment
Garcia Ruiz 2005 {published data only}
-
- Garcia Ruiz AJ, Leiva Fernandez F, Martos Crespo F. Cost‐effectivenessanalysis of tiotropium compared to ipratropium and salmeterol. Archivos de Bronconeumología 2005;41:242‐8. - PubMed
Geitona 2011 {published data only}
-
- Geitona M, Hatzikou M, Bania E. Economic evaluation of indacaterol versus tiotropium or formoterol for patients with moderate to severe COPD in Greece. Value in Health. 2011; Vol. ISPOR 14th Annual European Congress, Madrid, Spain.
Price 2011 {published data only}
-
- Price D, Gray A, Gale R, Asukai Y, Mungapen L, Lloyd A, et al. Cost‐utility analysis of indacaterol in Germany: a once‐daily maintenance bronchodilator for patients with COPD. Respiratory Medicine 2011;105(11):1635‐47. - PubMed
Sanz‐Martinez 2004 {published data only}
-
- Sanz‐Martinez H, Perez‐Maroto MT. Cost‐efficacy analysis of tiotropium versus ipratropium and salmeterol in the treatment of chronic obstructive pulmonary disease. Farmacia Aten Primaria 2004;2:72‐82.
Schramm 2005 {published data only}
-
- Schramm W, Haake D, Brandt A. Economic value of tiotropium in the treatment of chronic obstructive pulmonary disease. Praxis 2005;94(46):1803‐10. - PubMed
Additional references
Appleton 2006
Barr 2005
Beeh 2010
-
- Beeh KM, Beier J. The short, the long and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐9. - PubMed
Beier 2011
Berger 2008
-
- Berger WE, Nadel JA. Efficacy and safety of formoterol for the treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2008;102(2):173‐88. - PubMed
Boehringer Ingelheim 2010
-
- Boehringer Ingelheim. Tiotropium (SPIRIVA®) RESPIMAT®: Evaluation of fatal events. www.trials.boehringer‐ingelheim.com/res/trial/data/pdf/Pooled_Analysis_U10‐3255‐01.pdf 2010.
Borg 2004
-
- Borg S, Ericsson A, Wedzicha J, Gulsvik A, Lundback B, Donaldson GC, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value in Health 2004;7(2):153‐67. - PubMed
Calverley 2007
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. - PubMed
Casaburi 2002
-
- Casaburi R, Mahler DA, Jones PW, Wanner A, San Pedro G, ZuWallack RL, et al. A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. European Respiratory Journal 2002;19(2):217‐24. - PubMed
Chapman 2002
-
- Chapman KR, Arvidsson P, Chuchalin AG, Dhillon DP, Faurschou P, Goldstein RS, et al. The addition of salmeterol 50 microg bid to anticholinergic treatment in patients with COPD: a randomized, placebo controlled trial. Chronic obstructive pulmonary disease. Canadian Respiratory Journal 2002;9(3):178‐85. - PubMed
Cheyne 2012
-
- Cheyne L, Irvin‐Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009552] - DOI
clinicaltrials.gov
-
- National Institutes of Health. Comparison of indacaterol and tiotropium on lung function and related outcomes in patients with chronic obstructive pulmonary disease (COPD) (INVIGORATE). http://clinicaltrials.gov/ct2/show/NCT00845728?term=tiotropium+indacater... 2012.
Dahl 2010
-
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled β2‐agonist indacaterol versus twice‐daily formoterol in COPD. Thorax 2010;65(6):473. - PubMed
Donohue 2002
-
- Donohue JF, Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, et al. A 6‐month, placebo‐controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122(1):47‐55. - PubMed
Donohue 2011a
Drummond 1996
Dusser 2006
-
- Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. European Respiratory Journal 2006; Vol. 27, issue 3:547‐55. [CTG: NCT00274014] - PubMed
Effing 2007
GOLD
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD. http://www.goldcopd.com 2009.
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008], The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2008.
Hutchinson 2010
-
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medicine Journal 2010;40(5):364‐71. - PubMed
Johnson 1998
-
- Johnson M. The beta‐adrenoceptor. American Journal of Respiratory and Critical Care Medicine 1998;158(5 Pt 3):S146‐53. - PubMed
Jones 1997
-
- Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. American Journal of Respiratory and Critical Care Medicine 1997;155(4):1283‐9. - PubMed
Jones 2005
-
- Jones P. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2:75‐9. - PubMed
Karner 2011a
Karner 2011b
Karner 2012
Karner 2012a
Kornmann 2011
-
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. Once‐daily indacaterol versus twice‐daily salmeterol for COPD: a placebo‐controlled comparison. European Respiratory Journal 2011;37(2):273‐9. - PubMed
Lacasse 2006
Miravitlles 2002
-
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest 2002;121(5):1449‐55. - PubMed
Miravitlles 2003
-
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Costs of chronic bronchitis and COPD: a 1‐year follow‐up study. Chest 2003;123(3):784‐91. - PubMed
NICE
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease (updated). http://www.nice.org.uk/guidance/CG101 2012.
Niewoehner 2005
-
- Niewoehner DE, Rice K, Cote C, Paulson D, Cooper Jr JAD, Korducki L, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Annals of Internal Medicine 2005; Vol. 143, issue 5:317‐26. [CTG: NCT00274547] - PubMed
Oostenbrink 2004
-
- Oostenbrink JB, Rutten‐van Molken MP. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98(9):883‐91. - PubMed
Oostenbrink 2004a
-
- Oostenbrink JB, Rutten‐van Molken MP, Al MJ, Noord JA, Vincken W. One‐year cost‐effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. European Respiratory Journal 2004;23(2):241‐9. - PubMed
Philips 2004
-
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision‐analytic modelling in health technology assessment. Health Technology Assessment. 2004/09/14 2004; Vol. 8, issue 36:iii‐iv, ix‐xi, 1‐158. - PubMed
Proskocil 2005
-
- Proskocil BJ, Fryer AD. Beta2‐agonist and anticholinergic drugs in the treatment of lung disease. Proceedings of the American Thoracic Society 2005;2(4):305‐10; discussion 311‐2. - PubMed
Rennard 2001
-
- Rennard SI, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, et al. Use of a long‐acting inhaled beta2‐adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(5):1087‐92. - PubMed
Review Manager 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rodrigo 2008
-
- Rodrigo GJ, Nannini LJ, Rodríguez‐Roisin R. Safety of long‐acting β‐agonists in stable COPD. Chest 2008;133(5):1079‐87. - PubMed
Sears 2008
-
- Sears MR. Long‐acting bronchodilators in COPD. Chest 2008;133(5):1057‐8. - PubMed
Sin 2004
-
- Sin DD, Golmohammadi K, Jacobs P. Cost‐effectiveness of inhaled corticosteroids for chronic obstructive pulmonary disease according to disease severity. American Journal of Medicine 2004;116(5):325‐31. - PubMed
Spencer 2011
Stockley 2006
Tanaka 2005
-
- Tanaka Y, Horinouchi T, Koike K. New insights into beta‐adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clinical and Experimental Pharmacology and Physiology 2005;32(7):503‐14. - PubMed
Tashkin 2008
-
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. New England Journal Medicine 2008;359(15):1543‐54. - PubMed
van der Meer 2001
Vincken 2002
-
- Vincken W, Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, et al. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. European Respiratory Journal 2002;19(2):209‐16. - PubMed
Wallukat 2002
-
- Wallukat G. The beta‐adrenergic receptors. Herz 2002;27(7):683‐90. - PubMed
Welsh 2010
WHO
-
- World Health Organization. Chronic Respiratory Diseases. http://www.who.int.
Wilson 2000
-
- Wilson L, Devine EB, So K. Direct medical costs of chronic obstructive pulmonary disease: chronic bronchitis and emphysema. Respiratory Medicine 2000;94(3):204‐13. - PubMed
Yohannes 2011
-
- Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta‐analysis of clinically relevant outcomes. Respiratory Care 2011;56(4):477‐87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

