Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin
- PMID: 22972510
- PMCID: PMC3484168
- DOI: 10.22203/ecm.v024a14
Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin
Abstract
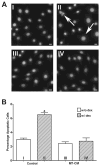
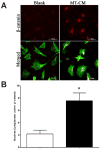
It is a widely held belief that the sole effect of muscle on bone is through mechanical loading. However, as the two tissues are intimately associated, we hypothesized that muscle myokines may have positive effects on bone. We found that factors produced by muscle will protect osteocytes from undergoing cell death induced by dexamethasone (dex), a glucocorticoid known to induce osteocyte apoptosis thereby compromising their capacity to regulate bone remodeling. Both the trypan blue exclusion assay for cell death and nuclear fragmentation assay for apoptosis were used. MLO-Y4 osteocytes, primary osteocytes, and MC3T3 osteoblastic cells were protected against dex-induced apoptosis by C2C12 myotube conditioned media (MT-CM) or by CM from ex vivo electrically stimulated, intact extensor digitorum longus (EDL) or soleus muscle derived from 4 month-old mice. C2C12 MT-CM, but not undifferentiated myoblast CM prevented dex-induced cell apoptosis and was potent down to 0.1 % CM. The CM from EDL muscle electrically stimulated tetanically at 80 Hz was more potent (10 fold) in prevention of dex-induced osteocyte death than CM from soleus muscle stimulated at the same frequency or CM from EDL stimulated at 1 Hz. This suggests that electrical stimulation increases production of factors that preserve osteocyte viability and that type II fibers are greater producers than type I fibers. The muscle factor(s) appears to protect osteocytes from cell death through activation of the Wnt/β-catenin pathway, as MT-CM induces β-catenin nuclear translocation and β-catenin siRNA abrogated the positive effects of MT-CM on dex-induced apoptosis. We conclude that muscle cells naturally secrete factor(s) that preserve osteocyte viability.
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures


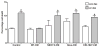


Similar articles
-
Muscle secreted factors enhance activation of the PI3K/Akt and β-catenin pathways in murine osteocytes.Bone. 2023 Sep;174:116833. doi: 10.1016/j.bone.2023.116833. Epub 2023 Jun 27. Bone. 2023. PMID: 37385426 Free PMC article.
-
Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the β-catenin and PKA pathways.J Bone Miner Res. 2010 Dec;25(12):2657-68. doi: 10.1002/jbmr.168. Epub 2010 Jun 24. J Bone Miner Res. 2010. PMID: 20578217 Free PMC article.
-
Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway.JBMR Plus. 2017 Oct;1(2):86-100. doi: 10.1002/jbm4.10015. Epub 2017 Oct 4. JBMR Plus. 2017. PMID: 29104955 Free PMC article.
-
Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications.Curr Osteoporos Rep. 2020 Feb;18(1):67-80. doi: 10.1007/s11914-020-00564-9. Curr Osteoporos Rep. 2020. PMID: 31953640 Review.
-
Wnt/β-Catenin Signaling in Craniomaxillofacial Osteocytes.Curr Osteoporos Rep. 2023 Apr;21(2):228-240. doi: 10.1007/s11914-023-00775-w. Epub 2023 Feb 21. Curr Osteoporos Rep. 2023. PMID: 36807035 Review.
Cited by
-
The osteocyte: an endocrine cell ... and more.Endocr Rev. 2013 Oct;34(5):658-90. doi: 10.1210/er.2012-1026. Epub 2013 Apr 23. Endocr Rev. 2013. PMID: 23612223 Free PMC article. Review.
-
β-catenin inhibition disrupts the homeostasis of osteogenic/adipogenic differentiation leading to the development of glucocorticoid-induced osteonecrosis of the femoral head.Elife. 2024 Feb 20;12:RP92469. doi: 10.7554/eLife.92469. Elife. 2024. PMID: 38376133 Free PMC article.
-
Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop.J Bone Miner Res. 2013 Sep;28(9):1857-65. doi: 10.1002/jbmr.1980. J Bone Miner Res. 2013. PMID: 23671010 Free PMC article. Review.
-
The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth.Int J Biochem Cell Biol. 2016 Aug;77(Pt A):23-29. doi: 10.1016/j.biocel.2016.05.015. Epub 2016 May 19. Int J Biochem Cell Biol. 2016. PMID: 27210503 Free PMC article. Review.
-
Fibroblast growth factor 9 (FGF9) inhibits myogenic differentiation of C2C12 and human muscle cells.Cell Cycle. 2019 Dec;18(24):3562-3580. doi: 10.1080/15384101.2019.1691796. Epub 2019 Nov 18. Cell Cycle. 2019. PMID: 31735119 Free PMC article.
References
-
- Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144:1761–1769. - PubMed
-
- Barclay CJ. Modelling diffusive O(2) supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil. 2005;26:225–235. - PubMed
-
- Beno T, Yoon YJ, Cowin SC, Fritton SP. Estimation of bone permeability using accurate microstructural measurements. J Biomech. 2006;39:2378–2387. - PubMed
-
- Bonewald LF. Osteocytes. In: Marcus R, Feldman D, Nelson D, Rosen C, editors. Osteoporosis. Elsevier; Amsterdam: 2007a. pp. 169–190.
-
- Bonewald LF. Osteocytes as dynamic, multifunctional cells. Ann N Y Acad Sci. 2007b;1116:281–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical