The combination of alisertib, an investigational Aurora kinase A inhibitor, and docetaxel promotes cell death and reduces tumor growth in preclinical cell models of upper gastrointestinal adenocarcinomas
- PMID: 22972611
- PMCID: PMC3524359
- DOI: 10.1002/cncr.27801
The combination of alisertib, an investigational Aurora kinase A inhibitor, and docetaxel promotes cell death and reduces tumor growth in preclinical cell models of upper gastrointestinal adenocarcinomas
Abstract
Background: Upper gastrointestinal adenocarcinomas (UGCs) respond poorly to current chemotherapeutic regimes. The authors and others have previously reported frequent Aurora kinase A (AURKA) gene amplification and mRNA and protein overexpression in UGCs. The objective of the current study was to determine the therapeutic potential of alisertib (MLN8237) alone and in combination with docetaxel in UGCs.
Methods: After treatment with alisertib and/or docetaxel, clonogenic cell survival, cell cycle analyses, Western blot analyses, and tumor xenograft growth assays were carried out to measure cell survival, cell cycle progression, apoptotic protein expression, and tumor xenograft volumes, respectively.
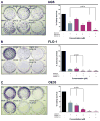
Results: By using the AGS, FLO-1, and OE33 UGC cell lines, which have constitutive AURKA overexpression and variable tumor protein 53 (p53) status, significantly enhanced inhibition of cancer cell survival was observed with alisertib and docetaxel treatment in combination (P < .001), compared with single-agent treatments. Cell cycle analyses, after 48 hours of treatment with alisertib, produced a significant increase in the percentage of polyploidy in UGC cells (P < .01) that was further enhanced by docetaxel (P < .001). In addition, an increase in the percentage of cells in sub-G1-phase observed with alisertib (P < .01) was significantly enhanced with the combination treatment (P < .001). Western blot analysis demonstrated higher induction of cleaved caspase 3 protein expression with the combined treatment compared with single-agent treatments. In addition, FLO-1 and OE33 cell xenograft models demonstrated enhanced antitumor activity for the alisertib and docetaxel combination compared with single-agent treatments (P < .001).
Conclusions: The current study demonstrated that alisertib combined with docetaxel can mediate a better therapeutic outcome in UGC cell lines.
Copyright © 2012 American Cancer Society.
Conflict of interest statement
Figures





Similar articles
-
Inhibition of AURKA Reduces Proliferation and Survival of Gastrointestinal Cancer Cells With Activated KRAS by Preventing Activation of RPS6KB1.Gastroenterology. 2019 Feb;156(3):662-675.e7. doi: 10.1053/j.gastro.2018.10.030. Epub 2018 Oct 17. Gastroenterology. 2019. PMID: 30342037 Free PMC article.
-
The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells.Mol Cancer Ther. 2012 Mar;11(3):763-74. doi: 10.1158/1535-7163.MCT-11-0623. Epub 2012 Feb 1. Mol Cancer Ther. 2012. PMID: 22302096 Free PMC article.
-
Targeting Aurora A kinase activity with the investigational agent alisertib increases the efficacy of cytarabine through a FOXO-dependent mechanism.Int J Cancer. 2012 Dec 1;131(11):2693-703. doi: 10.1002/ijc.27579. Epub 2012 Jun 28. Int J Cancer. 2012. PMID: 22488249 Free PMC article.
-
The role of alisertib in treatment of peripheral T-cell lymphomas.Future Oncol. 2015 Sep;11(18):2515-24. doi: 10.2217/fon.15.154. Epub 2015 Sep 7. Future Oncol. 2015. PMID: 26344156 Review.
-
Alisertib: a review of pharmacokinetics, efficacy and toxicity in patients with hematologic malignancies and solid tumors.Expert Opin Investig Drugs. 2018 Jan;27(1):105-112. doi: 10.1080/13543784.2018.1417382. Epub 2018 Jan 3. Expert Opin Investig Drugs. 2018. PMID: 29260599 Review.
Cited by
-
Inhibition of AURKA Reduces Proliferation and Survival of Gastrointestinal Cancer Cells With Activated KRAS by Preventing Activation of RPS6KB1.Gastroenterology. 2019 Feb;156(3):662-675.e7. doi: 10.1053/j.gastro.2018.10.030. Epub 2018 Oct 17. Gastroenterology. 2019. PMID: 30342037 Free PMC article.
-
Insights into the non-mitotic functions of Aurora kinase A: more than just cell division.Cell Mol Life Sci. 2020 Mar;77(6):1031-1047. doi: 10.1007/s00018-019-03310-2. Epub 2019 Sep 27. Cell Mol Life Sci. 2020. PMID: 31562563 Free PMC article. Review.
-
Identification of aurora kinase A as an unfavorable prognostic factor and potential treatment target for metastatic gastrointestinal stromal tumors.Oncotarget. 2014 Jun 30;5(12):4071-86. doi: 10.18632/oncotarget.1705. Oncotarget. 2014. PMID: 24901229 Free PMC article.
-
A phase I study of MK-5108, an oral aurora a kinase inhibitor, administered both as monotherapy and in combination with docetaxel, in patients with advanced or refractory solid tumors.Invest New Drugs. 2016 Feb;34(1):84-95. doi: 10.1007/s10637-015-0306-7. Epub 2015 Dec 1. Invest New Drugs. 2016. PMID: 26620496 Free PMC article. Clinical Trial.
-
The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells.Drug Des Devel Ther. 2015 Feb 17;9:1027-62. doi: 10.2147/DDDT.S74412. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25733818 Free PMC article.
References
-
- Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362(9380):305–15. - PubMed
-
- Reim D, Gertler R, Novotny A, et al. Adenocarcinomas of the Esophagogastric Junction Are More Likely to Respond to Preoperative Chemotherapy than Distal Gastric Cancer. Ann Surg Oncol - PubMed
-
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–50. - PubMed
-
- Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–53. - PubMed
-
- Cronin J, McAdam E, Danikas A, et al. Epidermal growth factor receptor (EGFR) is overexpressed in high-grade dysplasia and adenocarcinoma of the esophagus and may represent a biomarker of histological progression in Barrett’s esophagus (BE) Am J Gastroenterol. 106(1):46–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

