A novel imaging system permits real-time in vivo tumor bed assessment after resection of naturally occurring sarcomas in dogs
- PMID: 22972654
- PMCID: PMC3563778
- DOI: 10.1007/s11999-012-2560-8
A novel imaging system permits real-time in vivo tumor bed assessment after resection of naturally occurring sarcomas in dogs
Abstract
Background: Treatment of soft tissue sarcoma (STS) includes complete tumor excision. However, in some patients, residual sarcoma cells remain in the tumor bed. We previously described a novel hand-held imaging device prototype that uses molecular imaging to detect microscopic residual cancer in mice during surgery.
Questions/purposes: To test this device in a clinical trial of dogs with naturally occurring sarcomas, we asked: (1) Are any adverse clinical or laboratory effects observed after intravenous administration of the fluorescent probes? (2) Do canine sarcomas exhibit fluorescence after administration of the cathepsin-activated probe? (3) Is the tumor-to-background ratio sufficient to distinguish tumor from tumor bed? And (4) can residual fluorescence be detected in the tumor bed during surgery and does this correlate with a positive margin?
Methods: We studied nine dogs undergoing treatment for 10 STS or mast cell tumors. Dogs received an intravenous injection of VM249, a fluorescent probe that becomes optically active in the presence of cathepsin proteases. After injection, tumors were removed by wide resection. The tumor bed was imaged using the novel imaging device to search for residual fluorescence. We determined correlations between tissue fluorescence and histopathology, cathepsin protease expression, and development of recurrent disease. Minimum followup was 9 months (mean, 12 months; range, 9-15 months).
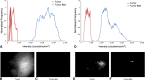
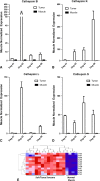
Results: Fluorescence was apparent from all 10 tumors and ranged from 3 × 10(7) to 1 × 10(9) counts/millisecond/cm(2). During intraoperative imaging, normal skeletal muscle showed no residual fluorescence. Histopathologic assessment of surgical margins correlated with intraoperative imaging in nine of 10 cases; in the other case, there was no residual fluorescence, but tumor was found at the margin on histologic examination. No animals had recurrent disease at 9 to 15 months.
Conclusions: These initial findings suggest this imaging system might be useful to intraoperatively detect residual tumor after wide resections.
Clinical relevance: The ability to assess the tumor bed intraoperatively for residual disease has the potential to improve local control.
Figures

Similar articles
-
A Novel Imaging System Distinguishes Neoplastic from Normal Tissue During Resection of Soft Tissue Sarcomas and Mast Cell Tumors in Dogs.Vet Surg. 2016 Aug;45(6):715-22. doi: 10.1111/vsu.12487. Epub 2016 Jun 9. Vet Surg. 2016. PMID: 27281113 Free PMC article.
-
Evaluation of a targeted anti-αvβ3 integrin near-infrared fluorescent dye for fluorescence-guided resection of naturally occurring soft tissue sarcomas in dogs.Eur J Nucl Med Mol Imaging. 2025 Feb;52(3):1137-1148. doi: 10.1007/s00259-024-06953-x. Epub 2024 Oct 22. Eur J Nucl Med Mol Imaging. 2025. PMID: 39436437 Free PMC article.
-
Long-term outcomes of dogs undergoing surgical resection of mast cell tumors and soft tissue sarcomas: A prospective 2-year-long study.Vet Surg. 2020 Jan;49(1):96-105. doi: 10.1111/vsu.13225. Epub 2019 May 2. Vet Surg. 2020. PMID: 31044443
-
Soft-tissue sarcomas in dogs: a review.J Am Anim Hosp Assoc. 2005 Jul-Aug;41(4):241-6. doi: 10.5326/0410241. J Am Anim Hosp Assoc. 2005. PMID: 15995161 Review.
-
Variability in tumor margin reporting for soft tissue sarcoma and cutaneous mast cell tumors in dogs: A systematic review.Vet Surg. 2021 Feb;50(2):259-272. doi: 10.1111/vsu.13539. Epub 2020 Dec 16. Vet Surg. 2021. PMID: 33331059
Cited by
-
A fibroblast activation protein targeted optical tracer for identifying primary and metastatic sarcoma during resection.EJNMMI Res. 2025 Jul 29;15(1):94. doi: 10.1186/s13550-025-01289-5. EJNMMI Res. 2025. PMID: 40728760 Free PMC article.
-
Intraoperative Raman spectroscopy of soft tissue sarcomas.Lasers Surg Med. 2016 Oct;48(8):774-781. doi: 10.1002/lsm.22564. Epub 2016 Jul 25. Lasers Surg Med. 2016. PMID: 27454580 Free PMC article. Clinical Trial.
-
The status of contemporary image-guided modalities in oncologic surgery.Ann Surg. 2015 Jan;261(1):46-55. doi: 10.1097/SLA.0000000000000622. Ann Surg. 2015. PMID: 25599326 Free PMC article. Review.
-
A Fluorescence-Guided Laser Ablation System for Removal of Residual Cancer in a Mouse Model of Soft Tissue Sarcoma.Theranostics. 2016 Jan 1;6(2):155-66. doi: 10.7150/thno.13536. eCollection 2016. Theranostics. 2016. PMID: 26877775 Free PMC article.
-
Intra-operative imaging of surgical margins of canine soft tissue sarcoma using optical coherence tomography.Vet Comp Oncol. 2019 Mar;17(1):80-88. doi: 10.1111/vco.12448. Epub 2018 Nov 13. Vet Comp Oncol. 2019. PMID: 30239117 Free PMC article.
References
-
- Allweis TM, Kaufman Z, Lelcuk S, Pappo I, Karni T, Schneebaum S, Spector R, Schindel A, Hershko D, Zilberman M, Sayfan J, Berlin Y, Hadary A, Olsha O, Paran H, Gutman M, Carmon M. A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg. 2008;196:483–489. doi: 10.1016/j.amjsurg.2008.06.024. - DOI - PubMed
-
- Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho A, Chiang DY, Reva B, Mermel CH, Getz G, Antipin Y, Beroukhim R, Major JE, Hatton C, Nicoletti R, Hanna M, Sharpe T, Fennell TJ, Cibulskis K, Onofrio RC, Saito T, Shukla N, Lau C, Nelander S, Silver SJ, Sougnez C, Viale A, Winckler W, Maki RG, Garraway LA, Lash A, Greulich H, Root DE, Sellers WR, Schwartz GK, Antonescu CR, Lander ES, Varmus HE, Ladanyi M, Sander C, Meyerson M, Singer S. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. - DOI - PMC - PubMed
-
- Ehrhart N. Soft-tissue sarcomas in dogs: a review. J Am Anim Hosp Assoc. 2005;41:241–246. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

