Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells
- PMID: 22972801
- PMCID: PMC3492830
- DOI: 10.1152/ajpcell.00175.2012
Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells
Abstract
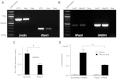
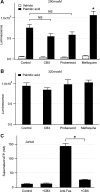
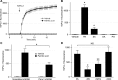
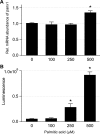
Hepatocyte lipoapoptosis induced by saturated free fatty acids (FFA) contributes to hepatic inflammation in lipotoxic liver injury, and the cellular mechanisms involved have not been defined. Recent studies have shown that apoptosis in nonhepatic cells stimulates ATP release via activation of pannexin1 (panx1), and extracellular ATP functions as a proinflammatory signal for recruitment and activation of the inflammatory cells. However, it is not known whether lipoapoptosis stimulates ATP release in liver cells. We found that lipoapoptosis induced by saturated FFA stimulated ATP release in liver cells that increased extracellular ATP concentration by more than fivefold above the values observed in healthy cells. This sustained pathophysiological ATP release was not dependent on caspase-3/7 activation. Inhibition of c-Jun NH(2)-terminal kinase (JNK), a key mediator of lipoapoptosis, with SP600125 blocked pathophysiological ATP release in a dose-dependent manner. RT-PCR analysis indicated that panx1 is expressed in hepatocytes and multiple liver cell lines. Notably, inhibition of panx1 expression with short hairpin (sh)RNA inhibited in part pathophysiological ATP release. Moreover, lipoapoptosis stimulated uptake of a membrane impermeable dye YoPro-1 (indicative of panx1 activation), which was inhibited by panx1 shRNA, probenecid, and mefloquine. These results suggest that panx1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated FFA. Thus panx1 may play an important role in hepatic inflammation by mediating an increase in extracellular ATP concentration in lipotoxic liver injury.
Figures


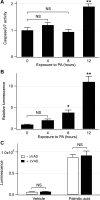
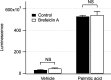
References
-
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297– 2307, 2006 - PubMed
-
- Bunse S, Locovei S, Schmidt M, Qiu F, Zoidl G, Dahl G, Dermietzel R. The potassium channel subunit Kvbeta3 interacts with pannexin 1 and attenuates its sensitivity to changes in redox potentials. FEBS J 276: 6258– 6270, 2009 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

