VDAC: old protein with new roles in diabetes
- PMID: 22972802
- PMCID: PMC3492836
- DOI: 10.1152/ajpcell.00087.2012
VDAC: old protein with new roles in diabetes
Abstract
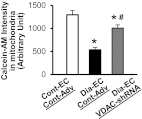
A decrease in capillary density due to an increase in endothelial cell apoptosis in the heart is implicated in cardiac ischemia in diabetes. The voltage-dependent anion channel (VDAC) plays a crucial role in the regulation of mitochondrial metabolic function and mitochondria-mediated apoptosis. This study is designed to examine the role of VDAC in coronary endothelial dysfunction in diabetes. Endothelial cells (ECs) were more apoptotic in diabetic left ventricle of diabetic mice and mouse coronary ECs (MCECs) isolated from diabetic mice exhibited significantly higher mitochondrial Ca(2+) concentration and VDAC protein levels than control MCECs. The expression of VDAC-short hairpin RNA (shRNA) not only decreased the resting mitochondrial Ca(2+) concentration but also attenuated mitochondrial Ca(2+) uptake in diabetic MCECs. Furthermore, the downregulation of VDAC in diabetic MCECs significantly decreased mitochondrial superoxide anion (O(2)(-)) production and the activity of the mitochondrial permeability transition pore (mPTP) opening (an indirect indicator of cell apoptosis) toward control levels. These data suggest that the increased VDAC level in diabetic MCECs is responsible for increased mitochondrial Ca(2+) concentration, mitochondrial O(2)(-) production, and mPTP opening activity. Normalizing VDAC protein level may help to decrease endothelial cell apoptosis, increase capillary density in the heart, and subsequently decrease the incidence of cardiac ischemia in diabetes.
Figures




Similar articles
-
Overexpression of hexokinase 2 reduces mitochondrial calcium overload in coronary endothelial cells of type 2 diabetic mice.Am J Physiol Cell Physiol. 2018 Jun 1;314(6):C732-C740. doi: 10.1152/ajpcell.00350.2017. Epub 2018 Mar 7. Am J Physiol Cell Physiol. 2018. PMID: 29513568 Free PMC article.
-
Mitochondrial connexin40 regulates mitochondrial calcium uptake in coronary endothelial cells.Am J Physiol Cell Physiol. 2017 Apr 1;312(4):C398-C406. doi: 10.1152/ajpcell.00283.2016. Epub 2017 Jan 25. Am J Physiol Cell Physiol. 2017. PMID: 28122731 Free PMC article.
-
Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes.Diabetologia. 2010 Aug;53(8):1783-94. doi: 10.1007/s00125-010-1770-4. Epub 2010 May 13. Diabetologia. 2010. PMID: 20461356 Free PMC article.
-
VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis.J Bioenerg Biomembr. 2008 Jun;40(3):199-205. doi: 10.1007/s10863-008-9142-1. J Bioenerg Biomembr. 2008. PMID: 18670869 Review.
-
A Calcium Guard in the Outer Membrane: Is VDAC a Regulated Gatekeeper of Mitochondrial Calcium Uptake?Int J Mol Sci. 2021 Jan 19;22(2):946. doi: 10.3390/ijms22020946. Int J Mol Sci. 2021. PMID: 33477936 Free PMC article. Review.
Cited by
-
Dysfunctional mitochondrial bioenergetics and oxidative stress in Akita(+/Ins2)-derived β-cells.Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E585-99. doi: 10.1152/ajpendo.00093.2013. Epub 2013 Jul 2. Am J Physiol Endocrinol Metab. 2013. PMID: 23820623 Free PMC article.
-
Dichloroacetate as a metabolic modulator of heart mitochondrial proteome under conditions of reduced oxygen utilization.Sci Rep. 2022 Sep 29;12(1):16348. doi: 10.1038/s41598-022-20696-5. Sci Rep. 2022. PMID: 36175475 Free PMC article.
-
The Mitochondrial Voltage-Dependent Anion Channel 1, Ca2+ Transport, Apoptosis, and Their Regulation.Front Oncol. 2017 Apr 10;7:60. doi: 10.3389/fonc.2017.00060. eCollection 2017. Front Oncol. 2017. PMID: 28443244 Free PMC article. Review.
-
VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress.Cell Stress. 2017 Oct;1(1):11-36. doi: 10.15698/cst2017.10.104. Epub 2017 Oct 1. Cell Stress. 2017. PMID: 30542671 Free PMC article.
-
Tubular Mas receptor mediates lipid-induced kidney injury.Cell Death Dis. 2021 Jan 21;12(1):110. doi: 10.1038/s41419-020-03375-z. Cell Death Dis. 2021. PMID: 33479200 Free PMC article.
References
-
- Ahmed M, Muhammed SJ, Kessler B, Salehi A. Mitochondrial proteome analysis reveals altered expression of voltage dependent anion channels in pancreatic beta-cells exposed to high glucose. Islets 2: 283–292, 2010 - PubMed
-
- Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatr Cardiol 32: 258–262, 2011 - PubMed
-
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301: 513–517, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

