Isolated duodenal exclusion increases energy expenditure and improves glucose homeostasis in diet-induced obese rats
- PMID: 22972837
- PMCID: PMC3517672
- DOI: 10.1152/ajpregu.00262.2012
Isolated duodenal exclusion increases energy expenditure and improves glucose homeostasis in diet-induced obese rats
Abstract
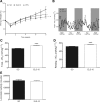
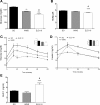
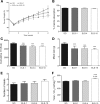
Roux-en-Y gastric bypass (RYGB) in rodent models reduces food intake (FI), increases resting energy expenditure (EE), and improves glycemic control. We have shown that mimicking the duodenal component of RYGB by implantation of a 10-cm endoluminal sleeve device (ELS-10) induces weight loss and improves glycemic control in diet-induced obese (DIO) rats. We sought to determine the mechanisms and structural requirements of these effects. We examined the effects of ELS-10 devices implanted in male DIO rats on body weight, food intake (FI), meal patterns, total and resting EE, and multiple parameters of glucose homeostasis, comparing them with sham-operated (SO) rats and with SO rats weight matched to the ELS-10-treated group. To determine the extent of duodenal exclusion required to influence metabolic outcomes, we compared the effects of implanting 10-, 4-, or 1-cm ELS devices. ELS-10 rats exhibited 13% higher total and 9% higher resting EE than SO controls. ELS-10 rats also exhibited enhanced postprandial GLP-1 secretion and improved glucose tolerance and insulin sensitivity out of proportion to the effects of weight loss alone. Implantation of 4- or 1-cm ELS devices had no effect on EE and limited effects on glucose homeostasis. Complete duodenal exclusion with ELS-10 induces weight loss by decreasing FI and increasing EE and improves glycemic control through weight loss-independent mechanisms. Thus signals originating in the proximal small intestine appear to exert a direct influence on the physiological regulation of EE and glucose homeostasis. Their selective manipulation could provide effective new therapies for obesity and diabetes that mimic the benefits of RYGB.
Figures


References
-
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724–1737, 2004 - PubMed
-
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248–256, 2009 - PubMed
-
- Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg 19: 1605–1611, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

