Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis
- PMID: 22972842
- PMCID: PMC4786373
- DOI: 10.1126/scitranslmed.3003783
Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis
Abstract
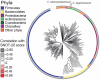
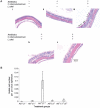
Persistent mucosal inflammation and microbial infection are characteristics of chronic rhinosinusitis (CRS). Mucosal microbiota dysbiosis is found in other chronic inflammatory diseases; however, the relationship between sinus microbiota composition and CRS is unknown. Using comparative microbiome profiling of a cohort of CRS patients and healthy subjects, we demonstrate that the sinus microbiota of CRS patients exhibits significantly reduced bacterial diversity compared with that of healthy controls. In our cohort of CRS patients, multiple, phylogenetically distinct lactic acid bacteria were depleted concomitant with an increase in the relative abundance of a single species, Corynebacterium tuberculostearicum. We recapitulated the conditions observed in our human cohort in a murine model and confirmed the pathogenic potential of C. tuberculostearicum and the critical necessity for a replete mucosal microbiota to protect against this species. Moreover, Lactobacillus sakei, which was identified from our comparative microbiome analyses as a potentially protective species, defended against C. tuberculostearicum sinus infection, even in the context of a depleted sinus bacterial community. These studies demonstrate that sinus mucosal health is highly dependent on the composition of the resident microbiota as well as identify both a new sino-pathogen and a strong bacterial candidate for therapeutic intervention.
Figures


References
-
- Ray NF, Baraniuk JN, Thamer M, Rinehart CS, Gergen PJ, Kaliner M, Josephs S, Pung YH. Healthcare expenditures for sinusitis in 1996: Contributions of asthma, rhinitis, and other airway disorders. J. Allergy Clin. Immunol. 1999;103:408–414. - PubMed
-
- Report of the Rhinosinusitis Task Force Committee Meeting Alexandria, Virginia, August 17, 1996. Otolaryngol. Head Neck Surg. 1997;117:S1–S68. - PubMed
-
- Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CC, Schuller D, Spector SL, Tilles SA. Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology, The diagnosis and management of rhinitis: An updated practice parameter. J. Allergy Clin. Immunol. 2008;122:S1–S84. - PubMed
-
- Biel MA, Brown CA, Levinson RM, Garvis GE, Paisner HM, Sigel ME, Tedford TM. Evaluation of the microbiology of chronic maxillary sinusitis. Ann. Otol. Rhinol. Laryngol. 1998;107:942–945. - PubMed
-
- Brook I, Frazier EH, Gher ME., Jr. Microbiology of periapical abscesses and associated maxillary sinusitis. J. Periodontol. 1996;67:608–610. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

