Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men
- PMID: 22972843
- PMCID: PMC3721979
- DOI: 10.1126/scitranslmed.3004006
Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men
Abstract
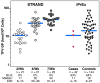
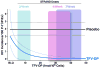
Drug concentrations associated with protection from HIV-1 acquisition have not been determined. We evaluated drug concentrations among men who have sex with men in a substudy of the iPrEx trial (1). In this randomized placebo-controlled trial, daily oral doses of emtricitabine/tenofovir disoproxil fumarate were used as pre-exposure prophylaxis (PrEP) in men who have sex with men. Drug was detected less frequently in blood plasma and in viable cryopreserved peripheral blood mononuclear cells (PBMCs) in HIV-infected cases at the visit when HIV was first discovered compared with controls at the matched time point of the study (8% versus 44%; P < 0.001) and in the 90 days before that visit (11% versus 51%; P < 0.001). An intracellular concentration of the active form of tenofovir, tenofovir-diphosphate (TFV-DP), of 16 fmol per million PBMCs was associated with a 90% reduction in HIV acquisition relative to the placebo arm. Directly observed dosing in a separate study, the STRAND trial, yielded TFV-DP concentrations that, when analyzed according to the iPrEx model, corresponded to an HIV-1 risk reduction of 76% for two doses per week, 96% for four doses per week, and 99% for seven doses per week. Prophylactic benefits were observed over a range of doses and drug concentrations, suggesting ways to optimize PrEP regimens for this population.
Conflict of interest statement
Figures


References
-
- UNAIDS. 2010
-
- Donnell D, et al. paper presented at the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. March 5–8 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

