Blockade of IL-10 signaling during bacillus Calmette-Guérin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-γ and IL-17 responses and increases protection to Mycobacterium tuberculosis infection
- PMID: 22972927
- PMCID: PMC3467194
- DOI: 10.4049/jimmunol.1201061
Blockade of IL-10 signaling during bacillus Calmette-Guérin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-γ and IL-17 responses and increases protection to Mycobacterium tuberculosis infection
Abstract
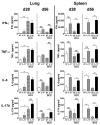
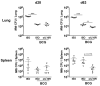
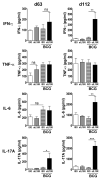
Vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG) remains the only prophylactic vaccine against tuberculosis, caused by Mycobacterium tuberculosis, but gives variable protection against pulmonary disease. The generation of host Th1 responses following BCG vaccination is accepted as the major mechanism of protection against M. tuberculosis infection. Early production of IL-17 in the lungs following M. tuberculosis challenge of mice previously vaccinated with M. tuberculosis peptides in adjuvant has been shown to be required for efficient Th1 cell recruitment. IL-10 regulates various processes involved in generation of Th1 and Th17 responses. Previous studies have shown IL-10 as a negative regulator of the immune response to primary M. tuberculosis infection, with Il10(-/-) mice having reduced lung bacterial loads. In this study we show that inhibition of IL-10 signaling during BCG vaccination enhances host-generated Ag-specific IFN-γ and IL-17A responses, and that this regimen gives significantly greater protection against aerogenic M. tuberculosis challenge in both susceptible and relatively resistant strains of mice. In M. tuberculosis-susceptible CBA/J mice, Ab blockade of IL-10R specifically during BCG vaccination resulted in additional protection against M. tuberculosis challenge of >1-log(10) compared with equivalent isotype-treated controls. The protection observed following BCG vaccination concurrent with anti-IL-10R mAb treatment was sustained through chronic M. tuberculosis infection and correlated with enhanced lung Th1 and Th17 responses and increased IFN-γ and IL-17A production by γδ T cells and an innate-like Thy1.2(+)CD3(-) lymphoid population. We show that IL-10 inhibits optimal BCG-elicited protection, therefore suggesting that antagonists of IL-10 may be of great benefit as adjuvants in preventive vaccination against tuberculosis.
Figures


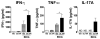

References
-
- W.H.O. Global Tuberculosis Control 2011. World Health Organisation; 2011.
-
- Dorman SE, Chaisson RE. From magic bullets back to the magic mountain: the rise of extensively drug-resistant tuberculosis. Nat Med. 2007;13:295–298. - PubMed
-
- Kaufmann SH. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2011;33:567–577. - PubMed
-
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

