Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency
- PMID: 22972948
- PMCID: PMC3500770
- DOI: 10.1136/jmedgenet-2012-101146
Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency
Abstract
Background: Isolated complex II deficiency is a rare form of mitochondrial disease, accounting for approximately 2% of all respiratory chain deficiency diagnoses. The succinate dehydrogenase (SDH) genes (SDHA, SDHB, SDHC and SDHD) are autosomally-encoded and transcribe the conjugated heterotetramers of complex II via the action of two known assembly factors (SDHAF1 and SDHAF2). Only a handful of reports describe inherited SDH gene defects as a cause of paediatric mitochondrial disease, involving either SDHA (Leigh syndrome, cardiomyopathy) or SDHAF1 (infantile leukoencephalopathy). However, all four SDH genes, together with SDHAF2, have known tumour suppressor functions, with numerous germline and somatic mutations reported in association with hereditary cancer syndromes, including paraganglioma and pheochromocytoma.
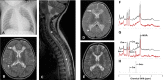
Methods and results: Here, we report the clinical and molecular investigations of two patients with histochemical and biochemical evidence of a severe, isolated complex II deficiency due to novel SDH gene mutations; the first patient presented with cardiomyopathy and leukodystrophy due to compound heterozygous p.Thr508Ile and p.Ser509Leu SDHA mutations, while the second patient presented with hypotonia and leukodystrophy with elevated brain succinate demonstrated by MR spectroscopy due to a novel, homozygous p.Asp48Val SDHB mutation. Western blotting and BN-PAGE studies confirmed decreased steady-state levels of the relevant SDH subunits and impairment of complex II assembly. Evidence from yeast complementation studies provided additional support for pathogenicity of the SDHB mutation.
Conclusions: Our report represents the first example of SDHB mutation as a cause of inherited mitochondrial respiratory chain disease and extends the SDHA mutation spectrum in patients with isolated complex II deficiency.
Figures




References
-
- Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain 2003;126:1905–12 - PubMed
-
- McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol 2010;9:829–40 - PubMed
-
- Rustin P, Rötig A. Inborn errors of complex II—unusual human mitochondrial diseases. Biochim Biophys Acta 2002;1553:117–22 - PubMed
-
- Parfait B, Chretien D, Rötig A, Marsac C, Munnich A, Rustin P. Compound heterozygous mutations in the flavoprotein gene of the respiratory chain complex II in a patient with Leigh syndrome. Hum Genet 2000;106:236–43 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
