Morphology, PKCδ expression, and synaptic responsiveness of different types of rat central lateral amygdala neurons
- PMID: 22972957
- PMCID: PMC3544890
- DOI: 10.1152/jn.00514.2012
Morphology, PKCδ expression, and synaptic responsiveness of different types of rat central lateral amygdala neurons
Abstract
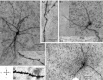
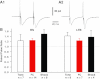
Recent findings implicate the central lateral amygdala (CeL) in conditioned fear. Indeed, CeL contains neurons exhibiting positive (CeL-On) or negative (CeL-Off) responses to fear-inducing conditioned stimuli (CSs). In mice, these cells differ in their expression of protein kinase Cδ (PKCδ) and physiological properties. CeL-Off cells are PKCδ(+) and late firing (LF), whereas CeL-On cells are PKCδ(-) and express a regular-spiking (RS) or low-threshold bursting (LTB) phenotype. However, the scarcity of LF cells in rats raises questions about the correspondence between the organization of CeL in mice and rats. Therefore, we studied the PKCδ expression, morphological properties, synaptic responsiveness, and fear conditioning-induced plasticity of rat CeL neurons. No PKCδ(+) LF cells were encountered, but ≈20-25% of RS and LTB neurons were PKCδ(+). Compared with RS neurons, a higher proportion of LTB cells projected to central medial amygdala (CeM) and they had fewer primary dendritic branches, yet the amplitude of excitatory postsynaptic potentials (EPSPs) evoked by lateral amygdala (LA) stimulation was similar in RS and LTB cells. In contrast, LA-evoked inhibitory postsynaptic potentials (IPSPs) had a higher amplitude in LTB than RS neurons. Finally, fear conditioning did not induce plasticity at LA inputs to RS or LTB neurons. These findings point to major species differences in the organization of CeL. Since rat LTB cells are subjected to stronger feedforward inhibition, they are more likely to exhibit inhibitory CS responses than RS cells. This is expected to cause a disinhibition of CeM fear output neurons and therefore an increase in fear expression.
Figures




References
-
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 329: 201–229, 1993 - PubMed
-
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdala circuits. Nature 468: 277–282, 2010 - PubMed
-
- Davis M. Pharmacological analysis of fear-potentiated startle. Braz J Med Biol Res 26: 235–260, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

