Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression
- PMID: 22972984
- PMCID: PMC3488886
- DOI: 10.1182/blood-2012-06-435362
Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression
Abstract
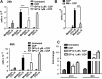
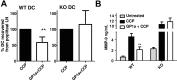
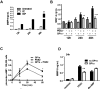
Administration of cannabinoid receptor 2 (CB2R) agonists in inflammatory and autoimmune disease and CNS injury models results in significant attenuation of clinical disease, and reduction of inflammatory mediators. Previous studies reported that CB2R signaling also reduces leukocyte migration. Migration of dendritic cells (DCs) to various sites is required for their activation and for the initiation of adaptive immune responses. Here, we report for the first time that CB2R signaling affects DC migration in vitro and in vivo, primarily through the inhibition of matrix metalloproteinase 9 (MMP-9) expression. Reduced MMP-9 production by DCs results in decreased migration to draining lymph nodes in vivo and in vitro in the matrigel migration assay. The effect on Mmp-9 expression is mediated through CB2R, resulting in reduction in cAMP levels, subsequent decrease in ERK activation, and reduced binding of c-Fos and c-Jun to Mmp-9 promoter activator protein 1 sites. We postulate that, by dampening production of MMP-9 and subsequent MMP-9-dependent DC migration, cannabinoids contribute to resolve acute inflammation and to reestablish homeostasis. Selective CB2R agonists might be valuable future therapeutic agents for the treatment of chronic inflammatory conditions by targeting activated immune cells, including DCs.
Figures




References
-
- Kubajewska I, Constantinescu CS. Cannabinoids and experimental models of multiple sclerosis. Immunobiology. 2010;215(8):647–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

