Excess F-actin mechanically impedes mitosis leading to cytokinesis failure in X-linked neutropenia by exceeding Aurora B kinase error correction capacity
- PMID: 22972986
- PMCID: PMC4338607
- DOI: 10.1182/blood-2012-03-419663
Excess F-actin mechanically impedes mitosis leading to cytokinesis failure in X-linked neutropenia by exceeding Aurora B kinase error correction capacity
Abstract
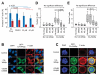
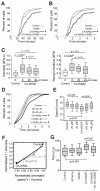
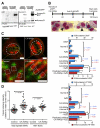
The constitutively active mutant of the Wiskott-Aldrich Syndrome protein (CA-WASp) is the cause of X-linked neutropenia and is linked with genomic instability and myelodysplasia. CA-WASp generates abnormally high levels of cytoplasmic F-actin through dysregulated activation of the Arp2/3 complex leading to defects in cell division. As WASp has no reported role in cell division, we hypothesized that alteration of cell mechanics because of increased F-actin may indirectly disrupt dynamic events during mitosis. Inhibition of the Arp2/3 complex revealed that excess cytoplasmic F-actin caused increased cellular viscosity, slowed all phases of mitosis, and perturbed mitotic mechanics. Comparison of chromosome velocity to the cytoplasmic viscosity revealed that cells compensated for increased viscosity by up-regulating force applied to chromosomes and increased the density of microtubules at kinetochores. Mitotic abnormalities were because of overload of the aurora signaling pathway as subcritical inhibition of Aurora in CA-WASp cells caused increased cytokinesis failure, while overexpression reduced defects. These findings demonstrate that changes in cell mechanics can cause significant mitotic abnormalities leading to genomic instability, and highlight the importance of mechanical sensors such as Aurora B in maintaining the fidelity of hematopoietic cell division.
Figures


References
-
- Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 2010;10(3):182–192. - PubMed
-
- Devriendt K, Kim AS, Mathijs G, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. NatGenet. 2001;27(3):313–317. - PubMed
-
- Ancliff PJ, Blundell MP, Cory GO, et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood. 2006;108(7):2182–2189. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

