Group I mGluR-mediated inhibition of Kir channels contributes to retinal Müller cell gliosis in a rat chronic ocular hypertension model
- PMID: 22972998
- PMCID: PMC6703809
- DOI: 10.1523/JNEUROSCI.1291-12.2012
Group I mGluR-mediated inhibition of Kir channels contributes to retinal Müller cell gliosis in a rat chronic ocular hypertension model
Abstract
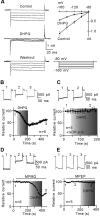
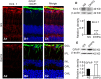
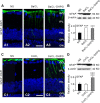
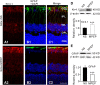
Müller cell gliosis, which is characterized by upregulated expression of glial fibrillary acidic protein (GFAP), is a universal response in many retinal pathological conditions. Whether down-regulation of inward rectifying K+ (Kir) channels, which commonly accompanies the enhanced GFAP expression, could contribute to Müller cell gliosis is poorly understood. We investigated changes of Kir currents, GFAP and Kir4.1 protein expression in Müller cells in a rat chronic ocular hypertension (COH) model, and explored the mechanisms underlying Müller cell gliosis. We show that Kir currents and Kir4.1 protein expression in Müller cells were reduced significantly, while GFAP expression was increased in COH rats, and these changes were eliminated by MPEP, a group I metabotropic glutamate receptors (mGluR I) subtype mGluR5 antagonist. In normal isolated Müller cells, the mGluR I agonist (S)-3,5-dihydroxyphenylglycine (DHPG) suppressed the Kir currents and the suppression was blocked by MPEP. The DHPG effect was mediated by the intracellular Ca2+ -dependent PLC/IP3-ryanodine/PKC signaling pathway, but the cAMP-PKA pathway was not involved. Moreover, intravitreal injection of DHPG in normal rats induced changes in Müller cells, similar to those observed in COH rats. The DHPG-induced increase of GFAP expression in Müller cells was obstructed by Ba2+, suggesting the involvement of Kir channels. We conclude that overactivation of mGluR5 by excessive extracellular glutamate in COH rats could contribute to Müller cell gliosis by suppressing Kir channels.
Figures






References
-
- Bolz S, Schuettauf F, Fries JE, Thaler S, Reichenbach A, Pannicke T. K+ currents fail to change in reactive retinal glial cells in a mouse model of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1249–1254. - PubMed
-
- Bringmann A, Reichenbach A. Role of Muller cells in retinal degenerations. Front Biosci. 2001;6:E72–E92. - PubMed
-
- Bringmann A, Francke M, Pannicke T, Biedermann B, Kodal H, Faude F, Reichelt W, Reichenbach A. Role of glial K+ channels in outogeny and gliosis: a hypothesis based upon studies on Müller cells. Glia. 2000;29:35–44. - PubMed
-
- Bringmann A, Pannicke T, Uhlmann S, Kohen L, Wiedemann P, Reichenbach A. Membrane conductance of Müller glial cells in proliferative diabetic retinopathy. Can J Ophthalmol. 2002a;37:221–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
