Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation
- PMID: 22973009
- PMCID: PMC3752079
- DOI: 10.1523/JNEUROSCI.2024-12.2012
Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation
Abstract
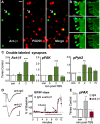
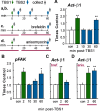
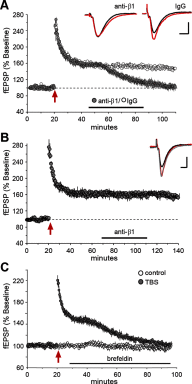
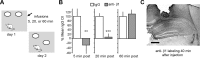
Memory consolidation theory posits that newly acquired information passes through a series of stabilization steps before being firmly encoded. We report here that in rat and mouse, hippocampus cell adhesion receptors belonging to the β1-integrin family exhibit dynamic properties in adult synapses and that these contribute importantly to a previously unidentified stage of consolidation. Quantitative dual immunofluorescence microscopy showed that induction of long-term potentiation (LTP) by theta burst stimulation (TBS) activates β1 integrins, and integrin-signaling kinases, at spine synapses in adult hippocampal slices. Neutralizing antisera selective for β1 integrins blocked these effects. TBS-induced integrin activation was brief (<7 min) and followed by an ∼45 min period during which the adhesion receptors did not respond to a second application of TBS. Brefeldin A, which blocks integrin trafficking to the plasma membrane, prevented the delayed recovery of integrin responses to TBS. β1 integrin-neutralizing antisera erased LTP when applied during, but not after, the return of integrin responsivity. Similarly, infusions of anti-β1 into rostral mouse hippocampus blocked formation of long-term, object location memory when started 20 min after learning but not 40 min later. The finding that β1 integrin neutralization was effective in the same time window for slice and behavioral experiments strongly suggests that integrin recovery triggers a temporally discrete, previously undetected second stage of consolidation for both LTP and memory.
Figures
References
-
- Bernard-Trifilo JA, Kramár EA, Torp R, Lin CY, Pineda EA, Lynch G, Gall CM. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem. 2005;93:834–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
