Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair
- PMID: 22973012
- PMCID: PMC3460536
- DOI: 10.1523/JNEUROSCI.2252-12.2012
Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair
Abstract
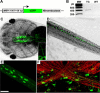
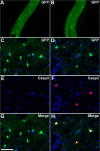
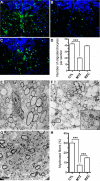
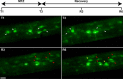
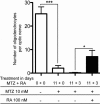
Live imaging studies of the processes of demyelination and remyelination have so far been technically limited in mammals. We have thus generated a Xenopus laevis transgenic line allowing live imaging and conditional ablation of myelinating oligodendrocytes throughout the CNS. In these transgenic pMBP-eGFP-NTR tadpoles the myelin basic protein (MBP) regulatory sequences, specific to mature oligodendrocytes, are used to drive expression of an eGFP (enhanced green fluorescent protein) reporter fused to the Escherichia coli nitroreductase (NTR) selection enzyme. This enzyme converts the innocuous prodrug metronidazole (MTZ) to a cytotoxin. Using two-photon imaging in vivo, we show that pMBP-eGFP-NTR tadpoles display a graded oligodendrocyte ablation in response to MTZ, which depends on the exposure time to MTZ. MTZ-induced cell death was restricted to oligodendrocytes, without detectable axonal damage. After cessation of MTZ treatment, remyelination proceeded spontaneously, but was strongly accelerated by retinoic acid. Altogether, these features establish the Xenopus pMBP-eGFP-NTR line as a novel in vivo model for the study of demyelination/remyelination processes and for large-scale screens of therapeutic agents promoting myelin repair.
Figures


References
-
- Bailey SM, Knox RJ, Hobbs SM, Jenkins TC, Mauger AB, Melton RG, Burke PJ, Connors TA, Hart IR. Investigation of alternative prodrugs for use with E. coli nitroreductase in ‘suicide gene’ approaches to cancer therapy. Gene Ther. 1996;3:1143–1150. - PubMed
-
- Baker D, Gerritsen W, Rundle J, Amor S. Critical appraisal of animal models of multiple sclerosis. Mult Scler. 2011;17:647–657. - PubMed
-
- Batoulis H, Recks MS, Addicks K, Kuerten S. Experimental autoimmune encephalomyelitis—achievements and prospective advances. APMIS. 2011;119:819–830. - PubMed
-
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
