Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia
- PMID: 22973013
- PMCID: PMC3499628
- DOI: 10.1523/JNEUROSCI.6451-11.2012
Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia
Abstract
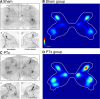
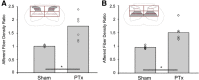
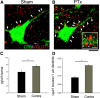
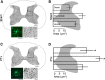
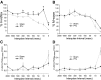
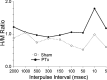
The corticospinal tract (CST) has dense contralateral and sparse ipsilateral spinal cord projections that converge with proprioceptive afferents on common spinal targets. Previous studies in adult rats indicate that the loss of dense contralateral spinal CST connections after unilateral pyramidal tract section (PTx), which models CST loss after stroke or spinal cord injury, leads to outgrowth from the spared side into the affected, ipsilateral, spinal cord. The reaction of proprioceptive afferents after this CST injury, however, is not known. Knowledge of proprioceptive afferent responses after loss of the CST could inform mechanisms of maladaptive plasticity in spinal sensorimotor circuits after injury. Here, we hypothesize that the loss of the contralateral CST results in a reactive increase in muscle afferents from the impaired limb and enhancement of their physiological actions within the cervical spinal cord. We found that 10 d after PTx, proprioceptive afferents sprout into cervical gray matter regions denervated by the loss of CST terminations. Furthermore, VGlut1-positive boutons, indicative of group 1A afferent terminals, increased on motoneurons. PTx also produced an increase in microglial density within the gray matter regions where CST terminations were lost. These anatomical changes were paralleled by reduction in frequency-dependent depression of the H-reflex, suggesting hyperreflexia. Our data demonstrate for the first time that selective CST injury induces maladaptive afferent fiber plasticity remote from the lesion. Our findings suggest a novel structural reaction of proprioceptive afferents to the loss of CST terminations and provide insight into mechanisms underlying spasticity.
Figures




Similar articles
-
Neuronal activity and microglial activation support corticospinal tract and proprioceptive afferent sprouting in spinal circuits after a corticospinal system lesion.Exp Neurol. 2019 Nov;321:113015. doi: 10.1016/j.expneurol.2019.113015. Epub 2019 Jul 18. Exp Neurol. 2019. PMID: 31326353 Free PMC article.
-
Competition with Primary Sensory Afferents Drives Remodeling of Corticospinal Axons in Mature Spinal Motor Circuits.J Neurosci. 2016 Jan 6;36(1):193-203. doi: 10.1523/JNEUROSCI.3441-15.2016. J Neurosci. 2016. PMID: 26740661 Free PMC article.
-
Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury.Exp Neurol. 2016 Mar;277:46-57. doi: 10.1016/j.expneurol.2015.12.008. Epub 2015 Dec 18. Exp Neurol. 2016. PMID: 26708732 Free PMC article.
-
Neuroplasticity of spinal cord injury and repair.Handb Clin Neurol. 2022;184:317-330. doi: 10.1016/B978-0-12-819410-2.00017-5. Handb Clin Neurol. 2022. PMID: 35034745 Review.
-
Harnessing neural activity to promote repair of the damaged corticospinal system after spinal cord injury.Neural Regen Res. 2016 Sep;11(9):1389-1391. doi: 10.4103/1673-5374.191199. Neural Regen Res. 2016. PMID: 27857728 Free PMC article. Review.
Cited by
-
Dual motor cortex and spinal cord neuromodulation improves rehabilitation efficacy and restores skilled locomotor function in a rat cervical contusion injury model.Exp Neurol. 2021 Jul;341:113715. doi: 10.1016/j.expneurol.2021.113715. Epub 2021 Apr 2. Exp Neurol. 2021. PMID: 33819448 Free PMC article.
-
EphA4-mediated ipsilateral corticospinal tract misprojections are necessary for bilateral voluntary movements but not bilateral stereotypic locomotion.J Neurosci. 2014 Apr 9;34(15):5211-21. doi: 10.1523/JNEUROSCI.4848-13.2014. J Neurosci. 2014. PMID: 24719100 Free PMC article.
-
Bilateral and asymmetrical contributions of passive and active ankle plantar flexors stiffness to spasticity in humans with spinal cord injury.J Neurophysiol. 2020 Sep 1;124(3):973-984. doi: 10.1152/jn.00044.2020. Epub 2020 May 20. J Neurophysiol. 2020. PMID: 32432501 Free PMC article.
-
Eccentric strengthening vs. conventional therapy in sub-acute stroke survivors: a randomized controlled trial.Front Neurol. 2025 Jan 23;15:1398860. doi: 10.3389/fneur.2024.1398860. eCollection 2024. Front Neurol. 2025. PMID: 39917437 Free PMC article.
-
Participatory design in the development of an early therapy intervention for perinatal stroke.BMC Pediatr. 2017 Jan 23;17(1):33. doi: 10.1186/s12887-017-0797-9. BMC Pediatr. 2017. PMID: 28114899 Free PMC article.
References
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. - PubMed
-
- Arendt-Nielsen L, Sonnenborg FA, Andersen OK. Facilitation of the withdrawal reflex by repeated transcutaneous electrical stimulation: an experimental study on central integration in humans. Eur J Appl Physiol. 2000;81:165–173. - PubMed
-
- Aymard C, Katz R, Lafitte C, Lo E, Pénicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123:1688–1702. - PubMed
-
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
