Reovirus uses multiple endocytic pathways for cell entry
- PMID: 22973022
- PMCID: PMC3497677
- DOI: 10.1128/JVI.01861-12
Reovirus uses multiple endocytic pathways for cell entry
Abstract
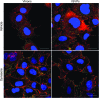
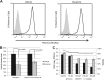
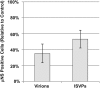
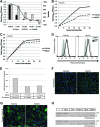
Entry of reovirus virions has been well studied in several tissue culture systems. After attachment to junctional adhesion molecule A (JAM-A), virions undergo clathrin-mediated endocytosis followed by proteolytic disassembly of the capsid and penetration to the cytoplasm. However, during in vivo infection of the intestinal tract, and likely in the tumor microenvironment, capsid proteolysis (uncoating) is initiated extracellularly. We used multiple approaches to determine if uncoated reovirus particles, called intermediate subviral particles (ISVPs), enter cells by directly penetrating the limiting membrane or if they take advantage of endocytic pathways to establish productive infection. We found that entry and infection by reovirus ISVPs was inhibited by dynasore, an inhibitor of dynamin-dependent endocytosis, as well as by genistein and dominant-negative caveolin-1, which block caveolar endocytosis. Inhibition of caveolar endocytosis also reduced infection by reovirus virions. Extraction of membrane cholesterol with methyl-β-cyclodextrin inhibited infection by virions but had no effect when infection was initiated with ISVPs. We found this pathway to be independent of both clathrin and caveolin. Together, these data suggest that reovirus virions can use both dynamin-dependent and dynamin-independent endocytic pathways during cell entry, and they reveal that reovirus ISVPs can take advantage of caveolar endocytosis to establish productive infection.
Figures


Similar articles
-
Interferon-inducible transmembrane protein 3 (IFITM3) restricts reovirus cell entry.J Biol Chem. 2013 Jun 14;288(24):17261-71. doi: 10.1074/jbc.M112.438515. Epub 2013 May 6. J Biol Chem. 2013. PMID: 23649619 Free PMC article.
-
Similar uptake but different trafficking and escape routes of reovirus virions and infectious subvirion particles imaged in polarized Madin-Darby canine kidney cells.Mol Biol Cell. 2013 Apr;24(8):1196-207. doi: 10.1091/mbc.E12-12-0852. Epub 2013 Feb 20. Mol Biol Cell. 2013. PMID: 23427267 Free PMC article.
-
Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein micro 1 mediates membrane disruption.J Virol. 2002 Oct;76(19):9920-33. doi: 10.1128/jvi.76.19.9920-9933.2002. J Virol. 2002. PMID: 12208969 Free PMC article.
-
Attachment and cell entry of mammalian orthoreovirus.Curr Top Microbiol Immunol. 2006;309:1-38. doi: 10.1007/3-540-30773-7_1. Curr Top Microbiol Immunol. 2006. PMID: 16909895 Review.
-
From touchdown to transcription: the reovirus cell entry pathway.Curr Top Microbiol Immunol. 2010;343:91-119. doi: 10.1007/82_2010_32. Curr Top Microbiol Immunol. 2010. PMID: 20397070 Free PMC article. Review.
Cited by
-
Interferon-inducible transmembrane protein 3 (IFITM3) restricts reovirus cell entry.J Biol Chem. 2013 Jun 14;288(24):17261-71. doi: 10.1074/jbc.M112.438515. Epub 2013 May 6. J Biol Chem. 2013. PMID: 23649619 Free PMC article.
-
Effects of Ipriflavone-Loaded Mesoporous Nanospheres on the Differentiation of Endothelial Progenitor Cells and Their Modulation by Macrophages.Nanomaterials (Basel). 2021 Apr 24;11(5):1102. doi: 10.3390/nano11051102. Nanomaterials (Basel). 2021. PMID: 33923311 Free PMC article.
-
Polymorphisms in the Most Oncolytic Reovirus Strain Confer Enhanced Cell Attachment, Transcription, and Single-Step Replication Kinetics.J Virol. 2020 Jan 31;94(4):e01937-19. doi: 10.1128/JVI.01937-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776267 Free PMC article.
-
Cleavage of the C-Terminal Fragment of Reovirus μ1 Is Required for Optimal Infectivity.J Virol. 2018 Feb 26;92(6):e01848-17. doi: 10.1128/JVI.01848-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298891 Free PMC article.
-
Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections.Viruses. 2020 Apr 26;12(5):487. doi: 10.3390/v12050487. Viruses. 2020. PMID: 32357558 Free PMC article. Review.
References
-
- Abulrob A, et al. 2004. Interactions of EGFR and caveolin-1 in human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene 23:6967–6979 - PubMed
-
- Aoki T, Nomura R, Fujimoto T. 1999. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp. Cell Res. 253:629–636 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

