The NS1 protein of influenza A virus interacts with cellular processing bodies and stress granules through RNA-associated protein 55 (RAP55) during virus infection
- PMID: 22973032
- PMCID: PMC3497642
- DOI: 10.1128/JVI.00647-12
The NS1 protein of influenza A virus interacts with cellular processing bodies and stress granules through RNA-associated protein 55 (RAP55) during virus infection
Abstract
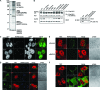
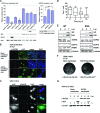
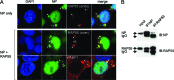
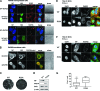
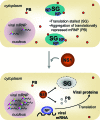
The nonstructural protein (NS1) of influenza A virus performs multiple functions in the virus life cycle. Proteomic screening for cellular proteins which interact with NS1 identified the cellular protein RAP55, which is one of the components of cellular processing bodies (P-bodies) and stress granules. To verify whether NS1 interacts with cellular P-bodies, interactions between NS1, RAP55, and other P-body-associated proteins (Ago1, Ago2, and DCP1a) were confirmed using coimmunoprecipitation and cellular colocalization assays. Overexpression of RAP55 induced RAP55-associated stress granule formation and suppressed virus replication. Knockdown of RAP55 with small interfering RNA (siRNA) or expression of a dominant-negative mutant RAP55 protein with defective interaction with P-bodies blocked NS1 colocalization to P-bodies in cells. Expression of NS1 inhibited RAP55 expression and formation of RAP55-associated P-bodies/stress granules. The viral nucleoprotein (NP) was found to be targeted to stress granules in the absence of NS1 but localized to P-bodies when NS1 was coexpressed. Restriction of virus replication via P-bodies occurred in the early phases of infection, as the number of RAP55-associated P-bodies in cells diminished over the course of virus infection. NS1 interaction with RAP55-associated P-bodies/stress granules was associated with RNA binding and mediated via a protein kinase R (PKR)-interacting viral element. Mutations introduced into either RNA binding sites (R38 and K41) or PKR interaction sites (I123, M124, K126, and N127) caused NS1 proteins to lose the ability to interact with RAP55 and to inhibit stress granules. These results reveal an interplay between virus and host during virus replication in which NP is targeted to P-bodies/stress granules while NS1 counteracts this host restriction mechanism.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

