Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia
- PMID: 22973054
- PMCID: PMC3484098
- DOI: 10.1091/mbc.E12-06-0424
Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia
Abstract
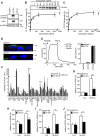
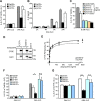
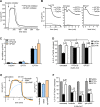
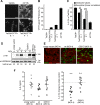
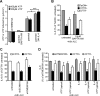
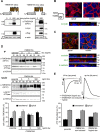
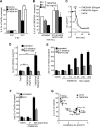
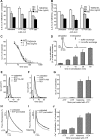
Cystic fibrosis (CF) is caused by the functional expression defect of the CF transmembrane conductance regulator (CFTR) chloride channel at the apical plasma membrane. Impaired bacterial clearance and hyperactive innate immune response are hallmarks of the CF lung disease, yet the existence of and mechanism accounting for the innate immune defect that occurs before infection remain controversial. Inducible expression of either CFTR or the calcium-activated chloride channel TMEM16A attenuated the proinflammatory cytokines interleukin-6 (IL-6), IL-8, and CXCL1/2 in two human respiratory epithelial models under air-liquid but not liquid-liquid interface culture. Expression of wild-type but not the inactive G551D-CFTR indicates that secretion of the chemoattractant IL-8 is inversely proportional to CFTR channel activity in cftr(∆F508/∆F508) immortalized and primary human bronchial epithelia. Similarly, direct but not P2Y receptor-mediated activation of TMEM16A attenuates IL-8 secretion in respiratory epithelia. Thus augmented proinflammatory cytokine secretion caused by defective anion transport at the apical membrane may contribute to the excessive and persistent lung inflammation in CF and perhaps in other respiratory diseases associated with documented down-regulation of CFTR (e.g., chronic obstructive pulmonary disease). Direct pharmacological activation of TMEM16A offers a potential therapeutic strategy to reduce the inflammation of CF airway epithelia.
Figures
References
-
- Accurso FJ, Moss RB, Wilmott RW, Anbar RD, Schaberg AE, Durham TA, Ramsey BW. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am J Respir Crit Care Med. 2011;183:627–634. - PubMed
-
- Adcock IM, Tsaprouni L, Bhavsar P, Ito K. Epigenetic regulation of airway inflammation. Curr Opin Immunol. 2007;19:694–700. - PubMed
-
- Anderson MP, Welsh MJ. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. - PubMed
-
- Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. 2005;40:500–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

