Comparative study of 1+PRN ranibizumab versus bevacizumab in the clinical setting
- PMID: 22973087
- PMCID: PMC3422156
- DOI: 10.2147/OPTH.S33017
Comparative study of 1+PRN ranibizumab versus bevacizumab in the clinical setting
Abstract
Purpose: We compared the efficacy of intravitreal ranibizumab and bevacizumab for treating neovascular age-related macular degeneration using an on-demand regimen.
Methods: A total of 186 wet age-related macular degeneration eyes of 186 treatment-naïve patients were compared retrospectively (67 eyes treated with ranibizumab with 91 treated with bevacizumab). At baseline, mean age, best corrected visual acuity, and angiographic lesion types were similar in both groups. Best corrected visual acuity and ocular coherence tomography were evaluated.
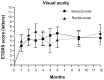
Results: Sixty eyes treated with ranibizumab and 85 eyes treated with bevacizumab completed a 12-month evaluation. At 12 months, mean best corrected visual acuity increased by +6.65 letters with ranibizumab treatment and by +5.59 with bevacizumab treatment (P = 0.64). Visual acuity improved by ≥15 letters in 15 eyes treated with ranibizumab and in 21 eyes treated with bevacizumab (P = 0.75). An overall reduction in ocular coherence tomography central thickness occurred for all time points. The mean number of injections per eye was 5.97 with ranibizumab and 5.92 with bevacizumab (P = 0.90).
Conclusion: Intravitreal therapies with ranibizumab or bevacizumab have similar visual and anatomical results. These results confirm those of comparison of Age-Related Macular Degeneration Treatment Trials in as-needed cohorts in clinical practice. Randomized long-term clinical trials are necessary to examine the systemic safety of these treatments.
Keywords: AMD; anti-VEGF therapy; bevacizumab; choroidal neovascularization; ranibizumab; wet AMD.
Figures


References
-
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004 Mar;137(3):486–495. - PubMed
-
- Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. - PubMed
-
- Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. - PubMed
-
- Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102(2):205–210. - PubMed
LinkOut - more resources
Full Text Sources

