Smooth muscle tension induces invasive remodeling of the zebrafish intestine
- PMID: 22973180
- PMCID: PMC3433428
- DOI: 10.1371/journal.pbio.1001386
Smooth muscle tension induces invasive remodeling of the zebrafish intestine
Abstract
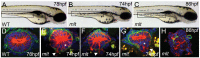
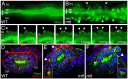
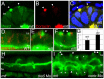
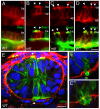
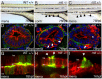
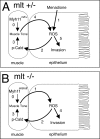
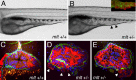
The signals that initiate cell invasion are not well understood, but there is increasing evidence that extracellular physical signals play an important role. Here we show that epithelial cell invasion in the intestine of zebrafish meltdown (mlt) mutants arises in response to unregulated contractile tone in the surrounding smooth muscle cell layer. Physical signaling in mlt drives formation of membrane protrusions within the epithelium that resemble invadopodia, matrix-degrading protrusions present in invasive cancer cells. Knockdown of Tks5, a Src substrate that is required for invadopodia formation in mammalian cells blocked formation of the protrusions and rescued invasion in mlt. Activation of Src-signaling induced invadopodia-like protrusions in wild type epithelial cells, however the cells did not migrate into the tissue stroma, thus indicating that the protrusions were required but not sufficient for invasion in this in vivo model. Transcriptional profiling experiments showed that genes responsive to reactive oxygen species (ROS) were upregulated in mlt larvae. ROS generators induced invadopodia-like protrusions and invasion in heterozygous mlt larvae but had no effect in wild type larvae. Co-activation of oncogenic Ras and Wnt signaling enhanced the responsiveness of mlt heterozygotes to the ROS generators. These findings present the first direct evidence that invadopodia play a role in tissue cell invasion in vivo. In addition, they identify an inducible physical signaling pathway sensitive to redox and oncogenic signaling that can drive this process.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Graded effects of unregulated smooth muscle myosin on intestinal architecture, intestinal motility and vascular function in zebrafish.Dis Model Mech. 2016 May 1;9(5):529-40. doi: 10.1242/dmm.023309. Epub 2016 Feb 18. Dis Model Mech. 2016. PMID: 26893369 Free PMC article.
-
Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine.Dev Cell. 2005 May;8(5):717-26. doi: 10.1016/j.devcel.2005.02.015. Dev Cell. 2005. PMID: 15866162
-
From Microspikes to Stress Fibers: Actin Remodeling in Breast Acini Drives Myosin II-Mediated Basement Membrane Invasion.Cells. 2021 Aug 4;10(8):1979. doi: 10.3390/cells10081979. Cells. 2021. PMID: 34440749 Free PMC article.
-
Biogenesis of invadopodia and their cellular functions.Postepy Biochem. 2014;60(1):62-8. Postepy Biochem. 2014. PMID: 25033543 Review.
-
Invadopodia and basement membrane invasion in vivo.Cell Adh Migr. 2014;8(3):246-55. doi: 10.4161/cam.28406. Cell Adh Migr. 2014. PMID: 24717190 Free PMC article. Review.
Cited by
-
Age-related changes in the cellular composition and epithelial organization of the mouse trachea.PLoS One. 2014 Mar 27;9(3):e93496. doi: 10.1371/journal.pone.0093496. eCollection 2014. PLoS One. 2014. PMID: 24675804 Free PMC article.
-
Assembly, heterogeneity, and breaching of the basement membranes.Cell Adh Migr. 2014;8(3):236-45. doi: 10.4161/cam.28733. Cell Adh Migr. 2014. PMID: 24727304 Free PMC article. Review.
-
Cdc42 and Tks5: a minimal and universal molecular signature for functional invadosomes.Cell Adh Migr. 2014;8(3):280-92. doi: 10.4161/cam.28833. Cell Adh Migr. 2014. PMID: 24840388 Free PMC article.
-
Graded effects of unregulated smooth muscle myosin on intestinal architecture, intestinal motility and vascular function in zebrafish.Dis Model Mech. 2016 May 1;9(5):529-40. doi: 10.1242/dmm.023309. Epub 2016 Feb 18. Dis Model Mech. 2016. PMID: 26893369 Free PMC article.
-
Integrins: Moonlighting Proteins in Invadosome Formation.Cancers (Basel). 2019 May 2;11(5):615. doi: 10.3390/cancers11050615. Cancers (Basel). 2019. PMID: 31052560 Free PMC article. Review.
References
-
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, et al. (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24–34. - PubMed
-
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, et al. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254. - PubMed
-
- Lehoux S, Tedgui A (2003) Cellular mechanics and gene expression in blood vessels. J Biomech 36: 631–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

