Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy
- PMID: 22973239
- PMCID: PMC3434369
- DOI: 10.3389/fphys.2012.00359
Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy
Abstract
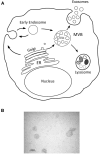
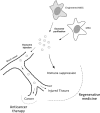
Mesenchymal stem cells (MSCs) are adult multipotent cells that give rise to various cell types of the mesodermal germ layer. MSCs are of great interest in the field of regenerative medicine and cancer therapy because of their unique ability to home to damaged and cancerous tissue. These cells also regulate the immune response and contribute to reparative processes in different pathological conditions, including musculoskeletal and cardiovascular diseases. The use of MSCs for tissue repair was initially based on the hypothesis that these cells home to and differentiate within the injured tissue into specialized cells. However, it now appears that only a small proportion of transplanted MSCs actually integrate and survive in host tissues. Thus, the predominant mechanism by which MSCs participate in tissue repair seems to be related to their paracrine activity. Indeed, MSCs provide the microenvironment with a multitude of trophic and survival signals including growth factors and cytokines. Recent discoveries suggest that lipid microvesicles released by MSCs may also be important in the physiological function of these cells. Over the past few years the biological relevance of micro- and nano-vesicles released by cells in intercellular communication has been established. Alongside the conventional mediators of cell secretome, these sophisticated nanovesicles transfer proteins, lipids and, most importantly, various forms of RNAs to neighboring cells, thereby mediating a variety of biological responses. The physiological role of MSC-derived vesicles (MSC-MVs) is currently not well understood. Nevertheless, encouraging results indicate that MSC-MVs have similar protective and reparative properties as their cellular counterparts in tissue repair and possibly anti-cancer therapy. Thus, MSC-MVs represent a promising opportunity to develop novel cell-free therapy approaches that might overcome the obstacles and risks associated with the use of native or engineered stem cells.
Keywords: exosomes; mesenchymal stem cell (MSC); microvesicles; regenerative medicine; therapy.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

