The Parasitic Dinoflagellates Blastodinium spp. Inhabiting the Gut of Marine, Planktonic Copepods: Morphology, Ecology, and Unrecognized Species Diversity
- PMID: 22973263
- PMCID: PMC3428600
- DOI: 10.3389/fmicb.2012.00305
The Parasitic Dinoflagellates Blastodinium spp. Inhabiting the Gut of Marine, Planktonic Copepods: Morphology, Ecology, and Unrecognized Species Diversity
Abstract
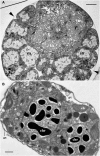
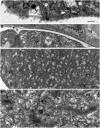
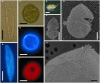
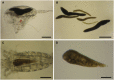
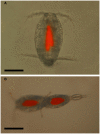
Blastodinium is a genus of dinoflagellates that live as parasites in the gut of marine, planktonic copepods in the World's oceans and coastal waters. The taxonomy, phylogeny, and physiology of the genus have only been explored to a limited degree and, based on recent investigations, we hypothesize that the morphological and genetic diversity within this genus may be considerably larger than presently recognized. To address these issues, we obtained 18S rDNA and ITS gene sequences for Blastodinium specimens of different geographical origins, including representatives of the type species. This genetic information was in some cases complemented with new morphological, ultrastructural, physiological, and ecological data. Because most current knowledge about Blastodinium and its effects on copepod hosts stem from publications more than half a century old, we here summarize and discuss the existing knowledge in relation to the new data generated. Most Blastodinium species possess functional chloroplasts, but the parasitic stage, the trophocyte, has etioplasts and probably a limited photosynthetic activity. Sporocytes and swarmer cells have well-developed plastids and plausibly acquire part of their organic carbon needs through photosynthesis. A few species are nearly colorless with no functional chloroplasts. The photosynthetic species are almost exclusively found in warm, oligotrophic waters, indicating a life strategy that may benefit from copepods as microhabitats for acquiring nutrients in a nutrient-limited environment. As reported in the literature, monophyly of the genus is moderately supported, but the three main groups proposed by Chatton in 1920 are consistent with molecular data. However, we demonstrate an important genetic diversity within the genus and provide evidences for new groups and the presence of cryptic species. Finally, we discuss the current knowledge on the occurrence of Blastodinium spp. and their potential impact on natural copepod populations.
Keywords: Blastodinium; copepod; parasite; phylogeny; plankton; symbiont; ultrastructure.
Figures






References
-
- Alves-de-Souza C., Cornet C., Nowaczyk A., Gasparini S., Skovgaard A., Guillou L. (2011). Blastodinium spp. infect copepods in the ultra-oligotrophic marine waters of the Mediterranean Sea. Biogeosciences 8, 2125–2136
-
- Apstein B. (1911). Parasiten von Calanus finmarchicus. Wiss. Meeresunters. Abt. Kiel 19, 206–223
-
- Chatton É. (1906). Les Blastodinides, ordre nouveuax de Dinoflagellés parasites. C. R. Acad. Sci. 144, 981–983
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

