Stromal cell contribution to human follicular lymphoma pathogenesis
- PMID: 22973275
- PMCID: PMC3433684
- DOI: 10.3389/fimmu.2012.00280
Stromal cell contribution to human follicular lymphoma pathogenesis
Abstract
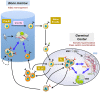
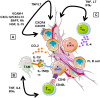
Follicular lymphoma (FL) is the prototypical model of indolent B cell lymphoma displaying a strong dependence on a specialized cell microenvironment mimicking normal germinal center. Within malignant cell niches in invaded lymph nodes and bone marrow, external stimuli provided by infiltrating stromal cells make a pivotal contribution to disease development, progression, and drug resistance. The crosstalk between FL B cells and stromal cells is bidirectional, causing activation of both partners. In agreement, FL stromal cells exhibit specific phenotypic, transcriptomic, and functional properties. This review highlights the critical pathways involved in the direct tumor-promoting activity of stromal cells but also their role in the organization of FL cell niche through the recruitment of accessory immune cells and their polarization to a B cell supportive phenotype. Finally, deciphering the interplay between stromal cells and FL cells provides potential new therapeutic targets with the aim to mobilize malignant cells outside their protective microenvironment and increase their sensitivity to conventional treatment.
Keywords: B cells; bone marrow; cell interactions; lymph nodes; stromal cells.
Figures
Similar articles
-
The yin and the yang of follicular lymphoma cell niches: role of microenvironment heterogeneity and plasticity.Semin Cancer Biol. 2014 Feb;24:23-32. doi: 10.1016/j.semcancer.2013.08.001. Epub 2013 Aug 23. Semin Cancer Biol. 2014. PMID: 23978491 Review.
-
IL-4/CXCL12 loop is a key regulator of lymphoid stroma function in follicular lymphoma.Blood. 2017 May 4;129(18):2507-2518. doi: 10.1182/blood-2016-08-737239. Epub 2017 Feb 15. Blood. 2017. PMID: 28202459
-
Integrative Analysis of Cell Crosstalk within Follicular Lymphoma Cell Niche: Towards a Definition of the FL Supportive Synapse.Cancers (Basel). 2020 Oct 5;12(10):2865. doi: 10.3390/cancers12102865. Cancers (Basel). 2020. PMID: 33028033 Free PMC article.
-
Bone Marrow Lymphoid Niche Adaptation to Mature B Cell Neoplasms.Front Immunol. 2021 Dec 8;12:784691. doi: 10.3389/fimmu.2021.784691. eCollection 2021. Front Immunol. 2021. PMID: 34956214 Free PMC article. Review.
-
The Supportive Role of Lymph Node Mesenchymal Stromal Cells in Follicular Lymphoma Involves the PITX1-hTERT-Podoplanin Axis.Stem Cells Dev. 2025 May;34(9-10):201-213. doi: 10.1089/scd.2025.0022. Epub 2025 Mar 25. Stem Cells Dev. 2025. PMID: 40130551
Cited by
-
The Roles of Mesenchymal Stromal/Stem Cells in Tumor Microenvironment Associated with Inflammation.Mediators Inflamm. 2016;2016:7314016. doi: 10.1155/2016/7314016. Epub 2016 Aug 18. Mediators Inflamm. 2016. PMID: 27630452 Free PMC article. Review.
-
Heat Shock Proteins in Lymphoma Immunotherapy.Front Immunol. 2021 Mar 18;12:660085. doi: 10.3389/fimmu.2021.660085. eCollection 2021. Front Immunol. 2021. PMID: 33815422 Free PMC article. Review.
-
T-Cell Clustering in Neoplastic Follicles of Follicular Lymphoma.Cancer Microenviron. 2018 Dec;11(2-3):135-140. doi: 10.1007/s12307-018-0217-1. Epub 2018 Sep 11. Cancer Microenviron. 2018. PMID: 30203411 Free PMC article.
-
Mesenchymal stromal cells support the viability and differentiation of follicular lymphoma-infiltrating follicular helper T-cells.PLoS One. 2014 May 16;9(5):e97597. doi: 10.1371/journal.pone.0097597. eCollection 2014. PLoS One. 2014. PMID: 24836297 Free PMC article.
-
Neutrophils trigger a NF-κB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas.Oncotarget. 2015 Jun 30;6(18):16471-87. doi: 10.18632/oncotarget.4106. Oncotarget. 2015. PMID: 26158216 Free PMC article.
References
-
- Amé-Thomas P., Le Priol J., Yssel H., Caron G., Pangault C., Jean R., Martin N., Marafioti T., Gaulard P., Lamy T., Fest T., Semana G., Tarte K. (2012). Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia 26, 1053–106310.1038/leu.2011.301 - DOI - PMC - PubMed
-
- Amé-Thomas P., Maby-El Hajjami H., Monvoisin C., Jean R., Monnier D., Caulet-Maugendre S., Guillaudeux T., Lamy T., Fest T., Tarte K. (2007). Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood 109, 693–70210.1182/blood-2006-05-020800 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

