Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes
- PMID: 22973278
- PMCID: PMC3438460
- DOI: 10.3389/fimmu.2012.00285
Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes
Abstract
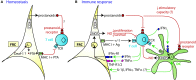
Fibroblastic reticular cells (FRC) form the structural backbone of the T cell rich zones in secondary lymphoid organs (SLO), but also actively influence the adaptive immune response. They provide a guidance path for immigrating T lymphocytes and dendritic cells (DC) and are the main local source of the cytokines CCL19, CCL21, and IL-7, all of which are thought to positively regulate T cell homeostasis and T cell interactions with DC. Recently, FRC in lymph nodes (LN) were also described to negatively regulate T cell responses in two distinct ways. During homeostasis they express and present a range of peripheral tissue antigens, thereby participating in peripheral tolerance induction of self-reactive CD8(+) T cells. During acute inflammation T cells responding to foreign antigens presented on DC very quickly release pro-inflammatory cytokines such as interferon γ. These cytokines are sensed by FRC which transiently produce nitric oxide (NO) gas dampening the proliferation of neighboring T cells in a non-cognate fashion. In summary, we propose a model in which FRC engage in a bidirectional crosstalk with both DC and T cells to increase the efficiency of the T cell response. However, during an acute response, FRC limit excessive expansion and inflammatory activity of antigen-specific T cells. This negative feedback loop may help to maintain tissue integrity and function during rapid organ growth.
Keywords: T lymphocyte activation; cyclooxygenase 2; fibroblastic reticular cell (FRC); immune tolerance; inducible nitric oxide synthase; lymph node stromal cells; mesenchymal stem cells; suppression.
Figures

References
-
- Albina J. E., Abate J. A., Henry W. L. (1991). Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J. Immunol. 147, 144–148 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

